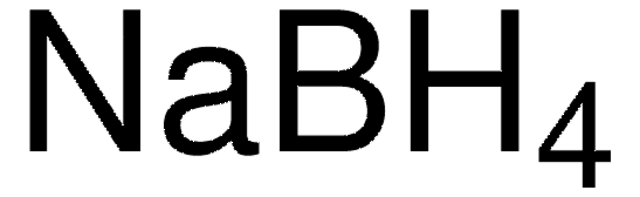추천 제품
Quality Level
분석
≥97.0% (gas-volumetric)
양식
solid
반응 적합성
reagent type: reductant
mp
125 °C (dec.) (lit.)
SMILES string
[Li].[AlH3]
InChI
1S/Al.Li.4H/q-1;+1;;;;
InChI key
OCZDCIYGECBNKL-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Lithium aluminum hydride (LiAlH4) is a powerful reducing agent used in organic synthesis to reduce esters, carboxylic acids, acyl chlorides, aldehydes, epoxides, and ketones into the corresponding alcohols. It also converts amide, nitro, nitrile, imine, oxime, and azide compounds into amines.
애플리케이션
Reactant involved in:
- Dehydrogenation studied with various catalysts
- Regeneration of methoxysilanes via reduction
- Transition metal electrophilic hydrogen activation via heterolytic cleavage
- Formation of nickel hydrogenation catalysts
- Hydrogen sorption
- Electrodeposition of Al on W-Cu substrates
포장
packed in plastic bag, which is soluble at 45-60°C in hydrocarbons, THF and dioxane
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1A - Water-react 1
Storage Class Code
4.3 - Hazardous materials which set free flammable gases upon contact with water
WGK
WGK 1
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
이미 열람한 고객
W. Han, et al.,
International Journal of Hydrogen Energy, 36, 12305-12312 (2011)
L. B. Belykh, et al.,
Applied Catalysis, 401, 65-72 (2011)
J. W. Runyon, et al.,
Australian Journal of Chemistry, 64, 1165-1172 (2011)
Rafi-ud-din, et al.,
The Journal of Physical Chemistry C, 115, 13088-13099 (2011)
R. A. Varin and L. Zbroniec,
J. Alloy Compounds, 509, S736-S739 (2011)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.