561487
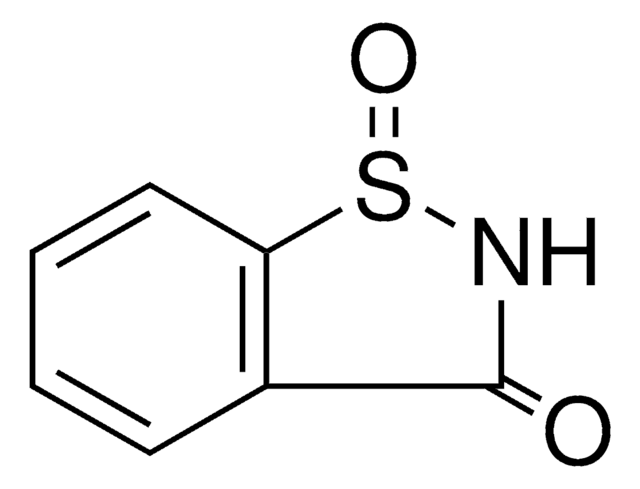
1,2-Benzisothiazol-3(2H)-one
97%
동의어(들):
1,2-Benzisothiazolone, 1,2-Benzoisothiazol-3-one, 1,2-benzothiazoline-3-one, 2,3-Dihydro-1,2-benzothiazol-3-one, 3-Hydroxy-1,2-benzisothiazole, Benzisothiazolin-3-one
로그인조직 및 계약 가격 보기
모든 사진(2)
About This Item
실험식(Hill 표기법):
C7H5NOS
CAS Number:
Molecular Weight:
151.19
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
분석
97%
양식
solid
mp
154-158 °C (lit.)
SMILES string
Oc1nsc2ccccc12
InChI
1S/C7H5NOS/c9-7-5-3-1-2-4-6(5)10-8-7/h1-4H,(H,8,9)
InChI key
DMSMPAJRVJJAGA-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
1,2-Benzisothiazol-3(2H)-one can be synthesized by reacting 2-mercaptobenzoic acid with diphenyl phosphoryl azide.
애플리케이션
1,2-Benzisothiazol-3(2H)-one can be used as a precursor in the synthesis of 1,2-benzoisothiazolin-3-one (BIT) derivative which can exhibit enhanced biological activities.
면책조항
The product is not intended for use as a biocide under global biocide regulations, including but not limited to US EPA′s Federal Insecticide Fungicide and Rodenticide Act, European Biocidal Products Regulation, Canada’s Pest Management Regulatory Agency, Turkey’s Biocidal Products Regulation, Korea’s Consumer Chemical Products and Biocide Safety Management Act (K-BPR) and others.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Eye Dam. 1 - Skin Irrit. 2 - Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
이미 열람한 고객
Convenient Synthesis of 1, 2-Benzisothiazol-3 (2H)-ones by Cyclization Reaction
of Acyl Azide
of Acyl Azide
Chiyoda T, et al.
Synlett, 2000(10), 1427-1428 (2000)
Mateo Alajarin et al.
The Journal of organic chemistry, 75(11), 3737-3750 (2010-05-14)
Under thermal activation in solution, N-[2-(1,3-oxathiolan-2-yl)]phenyl ketenimines and carbodiimides were converted into 2,1-benzisothiazol-3-ones bearing a pendant N-styryl or imidoyl fragment, respectively. These processes should occur with the concomitant formation of ethylene as result of the fragmentation of the 1,3-oxathiolane ring.
D L Greenway et al.
Letters in applied microbiology, 29(5), 298-302 (2000-02-09)
The effect of a commonly used biocide, 1,2-benzisothiazolin-3-one (BIT) on ppGpp accumulation in the pathogen, Pseudomonas aeruginosa PAO1, and an environmental isolate, Ps. fluorescens, was examined. It is concluded that BIT is able to induce a stringent response in Ps.
Pulpitis of the fingers from a shoe glue containing 1,2-benzisothiazolin-3-one (BIT).
M Ayadi et al.
Contact dermatitis, 40(2), 115-116 (1999-02-27)
D A Basketter et al.
Contact dermatitis, 40(3), 150-154 (1999-03-12)
Many of the chemicals in common use possess, to some degree, a capacity to cause skin sensitization. Consequently, it is important to conduct a thorough and accurate risk assessment when it can be anticipated that such chemicals are likely to
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.