추천 제품
Quality Level
분석
98%
refractive index
n20/D 1.506 (lit.)
bp
282 °C (lit.)
density
1.05 g/mL at 25 °C (lit.)
SMILES string
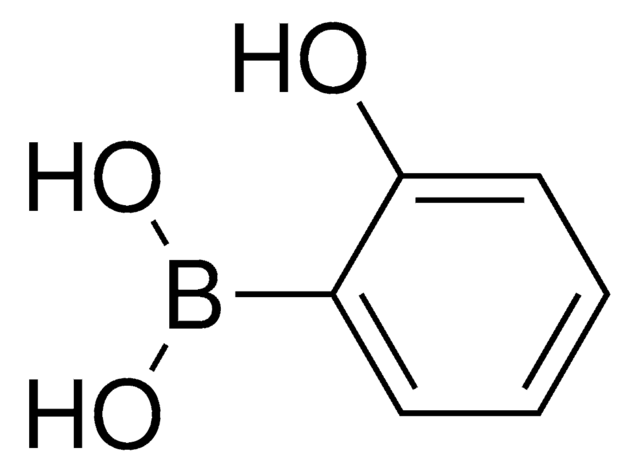
CC1(C)OB(OC1(C)C)c2ccccc2O
InChI
1S/C12H17BO3/c1-11(2)12(3,4)16-13(15-11)9-7-5-6-8-10(9)14/h5-8,14H,1-4H3
InChI key
VLROJECCXBBKPZ-UHFFFAOYSA-N
애플리케이션
Reactant involved in:
- Synthesis of indolo-fused heterocyclic inhibitors of polymerase enzyme of hepatitis C
- Studies of pi-interactions, electronic structure and transient UV absorption of subphthalocyanine-borate-bridged ferrocene-fullerene conjugates
- Synthesis of subphthalocyanine and fused-ring nicotine derivatives
- Suzuki-Miyaura coupling-triflation sequence, reduction and salt formation for synthesis of hydroxylated oligoarene phosphines
Widely used in Suzuki coupling chemistry.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
이미 열람한 고객
Tetrahedron, 58, 9633-9695 (2002)
문서
The synthesis of biaryl compounds via the Suzuki coupling reaction has become more commonplace now that many arylboronic acids are readily available. We are pleased to offer arylboronic acid pinacol esters4 as part of a growing line of products used in the Suzuki coupling reaction.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)


![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II), complex with dichloromethane](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)