모든 사진(2)
About This Item
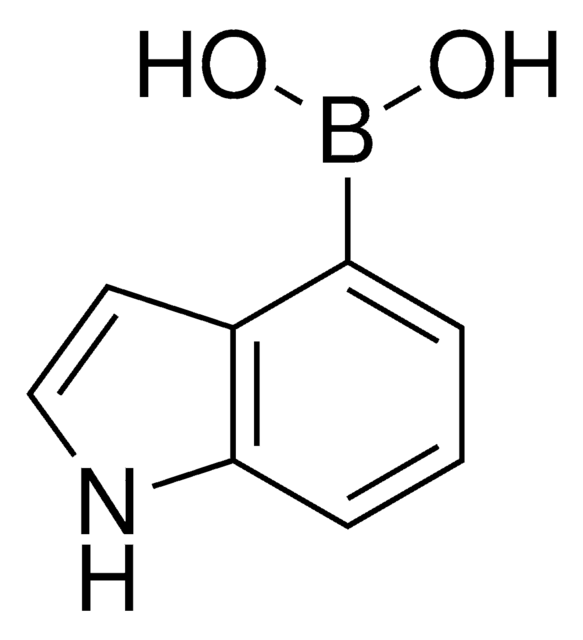
실험식(Hill 표기법):
C8H7BO2S
CAS Number:
Molecular Weight:
178.02
MDL number:
UNSPSC 코드:
12352103
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
≥95.0%
mp
225-230 °C (lit.)
SMILES string
OB(O)c1csc2ccccc12
InChI
1S/C8H7BO2S/c10-9(11)7-5-12-8-4-2-1-3-6(7)8/h1-5,10-11H
InChI key
QVANIYYVZZLQJP-UHFFFAOYSA-N
애플리케이션
Benzo[b]thien-3-ylboronic acid can be used:
- To prepare thienyl substituted pyrimidine derivatives as potent antimycobacterial compounds.
- To prepare 3-O-protected 17-heteroaryl-3-hydroxyestra-1,3,5,16-tetraene-16-carbaldehyde, which in turn is used for the synthesis of heteroarenes-annelated estranes.
- As a substrate in the study of metal-free coupling reactions of allylic alcohols with heteroaryl boronic acids.
- As a starting material for the preparation of thienyl based quinoline and pyridine ligands, which are further used to synthesize platinum complexes.
기타 정보
Contains varying amounts of anhydride
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
Synthesis, characterization, and photophysical properties of bismetalated platinum complexes with benzothiophene ligands
Anderson CM, et al.
Journal of Organometallic Chemistry, 882, 10-17 (2019)
Heteroareno-annelated estranes by triene cyclization
Watanabe M, et al.
open chemistry, 4(3), 375-402 (2006)
문서
This brochure contains a comprehensive selection of boronic acids, boronic acid esters, diboron esters, and transition-metal catalysts useful for the Suzuki–Miyaura coupling reaction
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.


![Benzo[b]thien-2-ylboronic acid ≥95%](/deepweb/assets/sigmaaldrich/product/structures/251/077/d0ead874-b533-4dcb-890d-8816a0018ccd/640/d0ead874-b533-4dcb-890d-8816a0018ccd.png)