480975
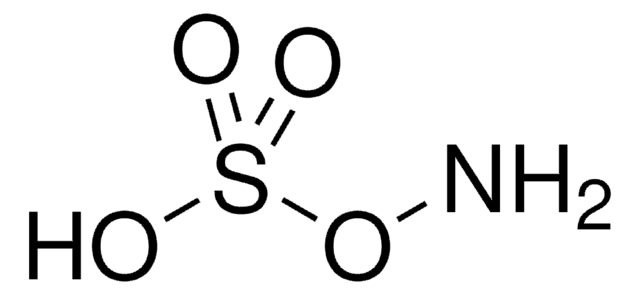
Hydroxylamine-O-sulfonic acid
99.998%
동의어(들):
(Aminooxy)(hydroxy)sulfane dioxide, (Aminooxy)sulfonic acid, Amidoperoxymonosulfuric acid, Amidosulfonic peracid, Aminomonopersulfuric acid, Ammonia-N-oxide-O-sulfonic acid betaine, Permonosulfamic acid, Sulfoperamidic acid
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
Linear Formula:
H2NOSO3H
CAS Number:
Molecular Weight:
113.09
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
99.998%
mp
210 °C (dec.) (lit.)
저장 온도
2-8°C
SMILES string
NOS(O)(=O)=O
InChI
1S/H3NO4S/c1-5-6(2,3)4/h1H2,(H,2,3,4)
InChI key
DQPBABKTKYNPMH-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Hydroxylamine-O-sulfonic acid (HOSA), an O-substituted hydroxylamine, is a widely used aminating agent. It can behave as a nucleophile as well as an electrophile. The conversion of organoboranes obtained from hydroboration of olefins to amines using HOSA has been reported.
애플리케이션
Hydroxylamine-O-sulfonic acid (HOSA) may be used to synthesize (Z)-1H-purin-6(7H)-ylideneaminooxysulfonic acid.
HOSA may be used as an aminating reagent in the synthesis of:
HOSA may be used as an aminating reagent in the synthesis of:
- 1-aminotetrazoles and 2-aminotetrazoles
- N-aminopiperidine (NAPP)
- 1H,1′ H-2,2′-biimidazole-1,1′-diamine
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 3 - Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
이미 열람한 고객
Hydroxylamine-O-sulfonic Acid.
Erdik E and Saczewski J.
e-EROS Encyclopedia of Reagents for Organic Synthesis. (2013)
A New Strategy for the Preparation of N-Aminopiperidine Using Hydroxylamine-O-Sulfonic Acid: Synthesis, Kinetic Modelling, Phase Equilibria, Extraction and Processes.
Labarthe E, et al.
Advances in Chemical Engineering and Science, 3(2) (2013)
N-Trinitroethylamino functionalization of nitroimidazoles: a new strategy for high performance energetic materials.
Yin P, et al.
Journal of Material Chemistry A, 1(25), 7500-7510 (2013)
Chen Zhu et al.
Journal of the American Chemical Society, 134(44), 18253-18256 (2012-10-23)
Herein, we disclose the first metal-free synthesis of primary aromatic amines from arylboronic acids, a reaction that has eluded synthetic chemists for decades. This remarkable transformation affords structurally diverse primary arylamines in good chemical yields, including a variety of halogenated
Synthesis and molecular structure of (Z)-1H-purin-6-ylideneaminooxysulfonic acid: a possible secondary metabolite of adenine.
Saczewski J and Gdaniec M.
Heterocyclic Communications, 18(3), 109-112 (2012)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.




![Tris[(1-benzyl-1H-1,2,3-triazol-4-yl)methyl]amine 97%](/deepweb/assets/sigmaaldrich/product/structures/179/695/86a721c8-2a4c-4e4f-bc36-6276ce7a941f/640/86a721c8-2a4c-4e4f-bc36-6276ce7a941f.png)



