모든 사진(2)
About This Item
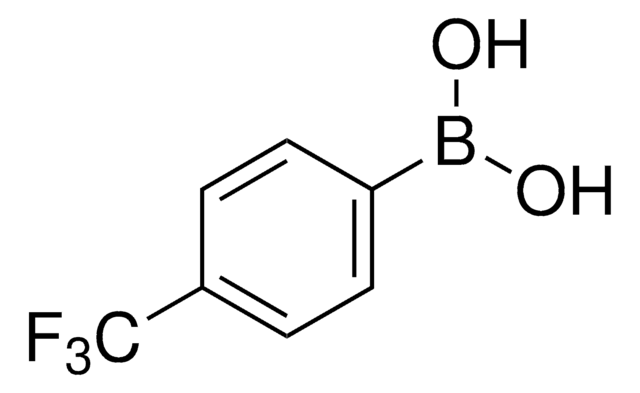
Linear Formula:
C10H7B(OH)2
CAS Number:
Molecular Weight:
171.99
Beilstein:
2936449
MDL number:
UNSPSC 코드:
12352103
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
≥95.0%
mp
269-275 °C (lit.)
SMILES string
OB(O)c1ccc2ccccc2c1
InChI
1S/C10H9BO2/c12-11(13)10-6-5-8-3-1-2-4-9(8)7-10/h1-7,12-13H
InChI key
KPTRDYONBVUWPD-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
Used in a study of an enantioselective rhodium-catalyzed addition of aryl boronic acids to 2,2,2-trifluoroacetophenones leading to chiral, tertiary trifluoromethyl alcohols. Also employed in a study of a palladium-catalyzed addition of aryl boronic acids to nitriles providing aryl ketones and to aryloxy nitriles providing benzofurans.
기타 정보
Contains varying amounts of anhydride
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
Sébastien L X Martina et al.
Chemical communications (Cambridge, England), (39), 4093-4095 (2006-10-07)
The catalytic asymmetric 1,2-addition of a series of arylboronic acids to 2,2,2-trifluoroacetophenones is described with high isolated yields (up to 96%) and good enantioselectivities (up to 83% ee) using a rhodium(I)/phosphoramidite catalyst.
Jae-Ryung Cha et al.
Journal of fluorescence, 24(4), 1215-1224 (2014-05-27)
Blue light-emitting spiro[benzotetraphene-fluorene] (SBTF)-based host materials, 3-(1-naphthyl)-10-naphthylspiro[benzo[ij]tetraphene-7,9'-fluorene] (1), 3-(2-naphthyl)-10-naphthylspiro[benzo[ij]tetraphene-7,9'-fluorene] (2), and 3-[2-(6-phenyl)naphthyl]-10-naphthylspiro[benzo[ij]tetraphene-7,9'-fluorene] (3) were designed and prepared via multi-step Suzuki coupling reactions. Introducing various aromatic groups into SBTF core lead to a reduction in band gap and a determination of
Saikat Das et al.
Chemphyschem : a European journal of chemical physics and physical chemistry, 18(1), 79-86 (2016-10-28)
Nuclear magnetic resonance is applied to investigate the relative positions and interactions between cationic and non-ionic carbohydrate-based surfactants in mixed micelles with D
Baowei Zhao et al.
Organic letters, 8(26), 5987-5990 (2006-12-15)
[Structure: see text] A cationic palladium complex catalyzed addition of arylboronic acids to nitriles to yield aryl ketones with moderate to good yields was developed. A one-step synthesis of benzofurans from phenoxyacetonitriles under the catalysis of [(bpy)Pd+(micro-OH)]2(-OTf)2 or [(bpy)Pd2+(H2O)2](-OTf)2 was
Alessio Innocenti et al.
Bioorganic & medicinal chemistry letters, 19(10), 2642-2645 (2009-04-21)
Inhibition of the beta-carbonic anhydrases (CAs, EC 4.2.1.1) from the pathogenic fungi Cryptococcus neoformans (Can2) and Candida albicans (Nce103) with a series of aromatic, arylalkenyl- and arylalkylboronic acids was investigated. Aromatic, 4-phenylsubstituted- and 2-naphthylboronic acids were the best Can2 inhibitors
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.