모든 사진(3)
About This Item
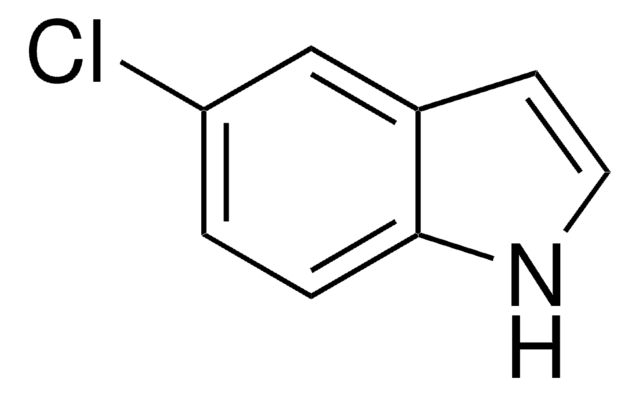
실험식(Hill 표기법):
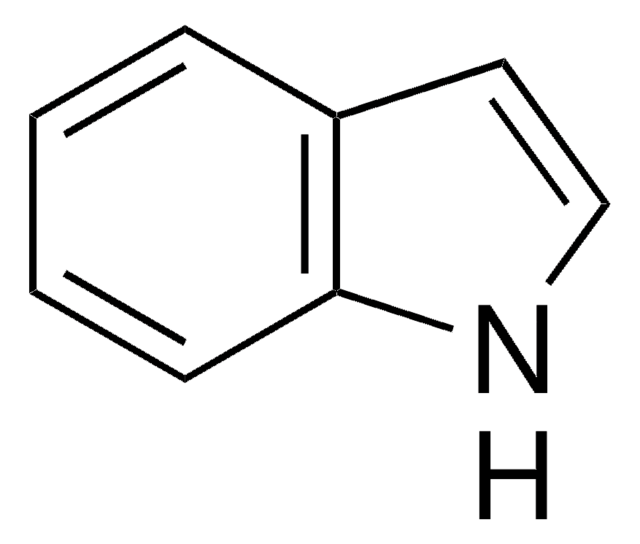
C8H6BrN
CAS Number:
Molecular Weight:
196.04
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
96%
양식
solid
mp
41-44 °C (lit.)
작용기
bromo
SMILES string
Brc1cccc2cc[nH]c12
InChI
1S/C8H6BrN/c9-7-3-1-2-6-4-5-10-8(6)7/h1-5,10H
InChI key
RDSVSEFWZUWZHW-UHFFFAOYSA-N
일반 설명
7-Bromoindole is a 7-substituted indole derivative. Its synthesis from 7-bromoindole-2-carboxylic acid has been reported. It has been reported to reduce the production of staphyloxanthin in Staphylococcus aureus.
애플리케이션
7-Bromoindole may be used in the synthesis of the following:
- indole
- dyestuffs
- 8-bromocarboline
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
235.4 °F - closed cup
Flash Point (°C)
113 °C - closed cup
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
Jin-Hyung Lee et al.
Applied microbiology and biotechnology, 97(10), 4543-4552 (2013-01-16)
Human pathogens can readily develop drug resistance due to the long-term use of antibiotics that mostly inhibit bacterial growth. Unlike antibiotics, antivirulence compounds diminish bacterial virulence without affecting cell viability and thus, may not lead to drug resistance. Staphylococcus aureus
Zhiqian Wang et al.
Tetrahedron letters, 53(5), 477-479 (2012-05-01)
A novel MCAP-cycloaddition sequence has been applied to the facile synthesis of β-carboline intermediates to gain rapid access to novel derivatives of yohimbine-like and corynanthe-like compounds that may be easily diversified by cross-coupling reactions and N-derivatizations to generate small compound
The structure of monobrominated ethyl indole-3-carboxylate and the preparation of 7-bromoindole.
Leggetter BE and Brown RK.
Canadian Journal of Chemistry, 38(9), 1467-1471 (1960)
Total synthesis of indoles from Tricholoma species via Bartoli/heteroaryl radical methodologies.
A Dobbs
The Journal of organic chemistry, 66(2), 638-641 (2001-06-30)
J Y Kim et al.
Letters in applied microbiology, 41(2), 163-168 (2005-07-22)
To establish multicomponent phenol hydroxylases (mPHs) as novel biocatalysts for producing dyestuffs and hydroxyindoles such as 7-hydroxyindole (7-HI) from indole and its derivatives. We have isolated Pseudomonas sp. KL33, which possesses a phenol degradation pathway similar to that found in
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.