추천 제품
mp
110-115 °C (lit.)
Quality Level
SMILES string
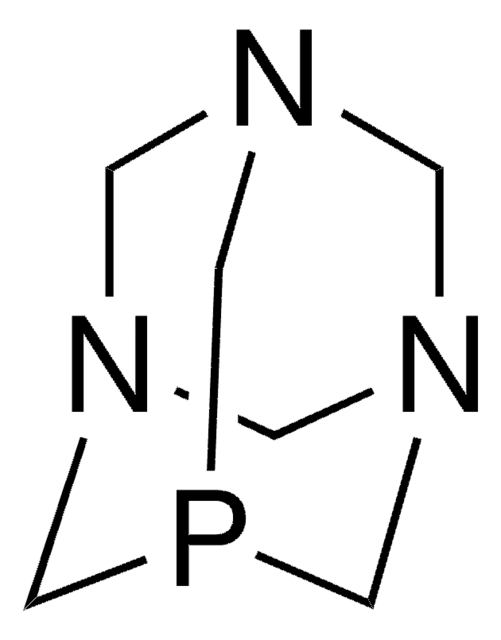
CN1CCN2CCN(C)P1N(C)CC2
InChI
1S/C9H21N4P/c1-10-4-7-13-8-5-11(2)14(10)12(3)6-9-13/h4-9H2,1-3H3
InChI key
PCYSWBQHCWWSFW-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
2,8,9-Trimethyl-2,5,8,9-tetraaza-1-phosphabicyclo[3.3.3]undecane is involved as a reactant in:
- Studies of N-heterocyclic carbene ligand effects on metal hydride bond energies.
- Synthesis of heterogenous basic catalysts immobilized on SBA-15 silica.
- Protection / deprotection strategies for diazeniumdiolate chemistry.
- Encaging of the molecule to study the change in its catalytic ability.
- C-N coupling reactions.
- Derivative synthesis used as a promoter for aza and thia-Michael reaction and Strecker reaction.
Reactant involved in:
- Studies of N-heterocyclic carbene ligand effects on metal hydride bond energies
- Synthesis of heterogenous basic catalysts immobilized on SBA-15 silica
- Protection / deprotection strategies for diazeniumdiolate chemistry
- Encaging of the molecule to study change in its catalytic ability
- C-N coupling reactions
- Derivative synthesis used as a promoter for aza and thia-Michael reaction and Strecker reaction
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
A new step towards solid base catalysis: azidoproazaphosphatranes immobilized in nanopores of mesoporous silica.
Raytchev P D, et al.
Advanced Synthesis & Catalysis, 353(11?12), 2067-2077 (2011)
Novel protection?deprotection strategies in diazeniumdiolate chemistry: synthesis of V-IPA/NO.
Nandurdikar R S, et al.
Chemical Communications (Cambridge, England), 47(23), 6710-6712 (2011)
Comprehensive Thermochemistry of W?H Bonding in the Metal Hydrides CpW (CO) 2 (IMes) H,[CpW (CO) 2 (IMes) H]?+, and [CpW (CO) 2 (IMes)(H) 2]+. Influence of an N-Heterocyclic Carbene Ligand on Metal Hydride Bond Energies.
Roberts J A, et al.
Journal of the American Chemical Society, 133(37), 14604-14613 (2011)
(t-Bu) 2PN P (i-BuNCH2CH2) 3N: New Efficient Ligand for Palladium-Catalyzed C? N Couplings of Aryl and Heteroaryl Bromides and Chlorides and for Vinyl Bromides at Room Temperature.
Reddy C V, et al.
The Journal of Organic Chemistry, 73(8), 3047-3062 (2008)
Encaging the Verkade?s superbases: thermodynamic and kinetic consequences.
Raytchev P D, et al.
Journal of the American Chemical Society, 133(7), 2157-2159 (2011)
문서
An article on Proazaphosphatranes: Verkade’s Superbases.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.![2,8,9-Triisopropyl-2,5,8,9-tetraaza-1-phosphabicyclo[3,3,3]undecane](/deepweb/assets/sigmaaldrich/product/structures/387/021/edaffe12-6e4b-4305-9030-749551ac828a/640/edaffe12-6e4b-4305-9030-749551ac828a.png)
![2,8,9-Triisobutyl-2,5,8,9-tetraaza-1-phosphabicyclo[3.3.3]undecane 97%](/deepweb/assets/sigmaaldrich/product/structures/750/287/cc77a98e-fa6c-4d81-9f3e-f392770724ac/640/cc77a98e-fa6c-4d81-9f3e-f392770724ac.png)



![2,8,9-Trimethyl-2,5,8,9-tetraaza-1-phosphabicyclo[3.3.3]undecane hydrochloride 96%](/deepweb/assets/sigmaaldrich/product/structures/212/000/1e518dee-a971-42c1-affc-372525c41bd0/640/1e518dee-a971-42c1-affc-372525c41bd0.png)
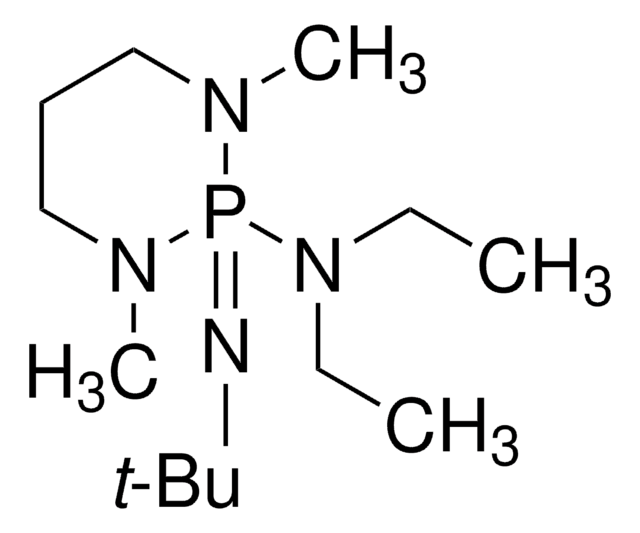

![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)