About This Item
추천 제품
Quality Level
분석
97%
양식
solid
반응 적합성
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Cross Couplings
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
core: palladium
mp
280 °C (dec.) (lit.)
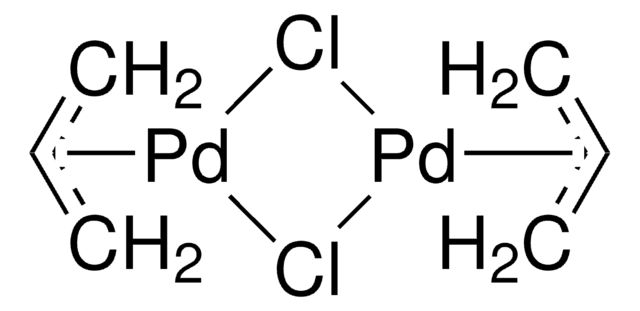
SMILES string
Cl[Pd]Cl.Cc1ccccc1P(c2ccccc2C)c3ccccc3C.Cc4ccccc4P(c5ccccc5C)c6ccccc6C
InChI
1S/2C21H21P.2ClH.Pd/c2*1-16-10-4-7-13-19(16)22(20-14-8-5-11-17(20)2)21-15-9-6-12-18(21)3;;;/h2*4-15H,1-3H3;2*1H;/q;;;;+2/p-2
InChI key
OTYPIDNRISCWQY-UHFFFAOYSA-L
일반 설명
애플리케이션
Catalyst for C-C and C-N coupling reaction.
- Reaction of tributyltin enolates, prepared in situ from tributyltin methoxide and enol acetates, with aryl bromides.
- Coupling reaction of aryl bromides with vinylic acetates.
- Negishi-Reformatsky coupling reaction of aryl bromides with ethyl 2-(tributylstannyl)acetates.
- Synthesis of (E)-methyl 3-(7-indolyl)-2-methacrylate, via Heck reaction.
- Synthesis of imidazopyrimidine derivatives.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
문서
The Heck reaction is the palladium catalyzed cross-coupling reaction between alkenes and aryl or vinyl halides (or triflates) to afford substituted alkenes.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.




![(R)-1-[(SP)-2-(Dicyclohexylphosphino)ferrocenyl]ethyldi-tert-butylphosphine ≥97%](/deepweb/assets/sigmaaldrich/product/structures/809/974/e027b628-7c2e-4bde-be7e-f9298d0c8b04/640/e027b628-7c2e-4bde-be7e-f9298d0c8b04.png)

![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)
![Dichloro[bis(2-(diphenylphosphino)phenyl)ether]palladium(II) 95%](/deepweb/assets/sigmaaldrich/product/structures/311/408/0ee427f0-19c0-413a-8f38-827359ddbcac/640/0ee427f0-19c0-413a-8f38-827359ddbcac.png)