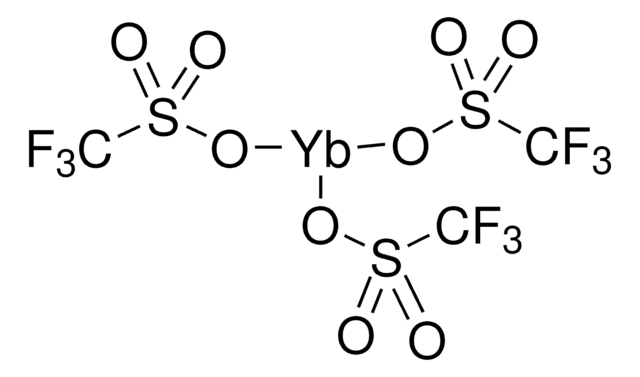
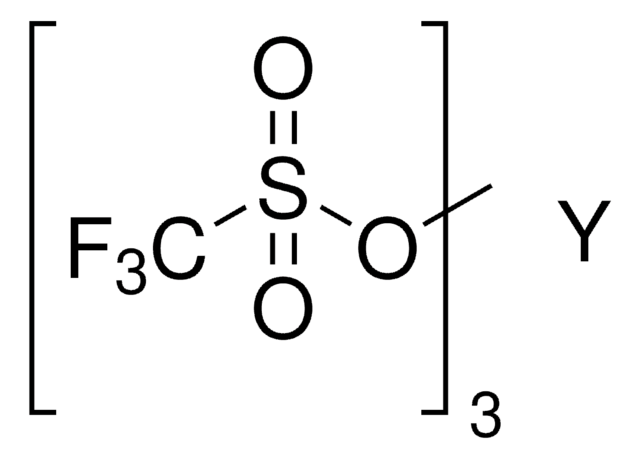
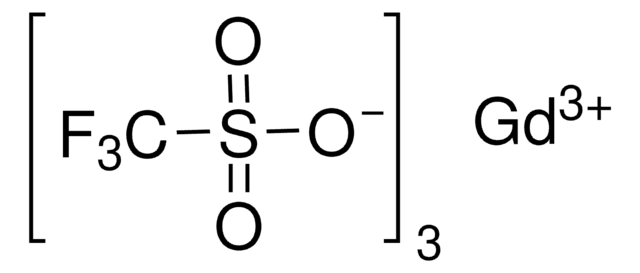
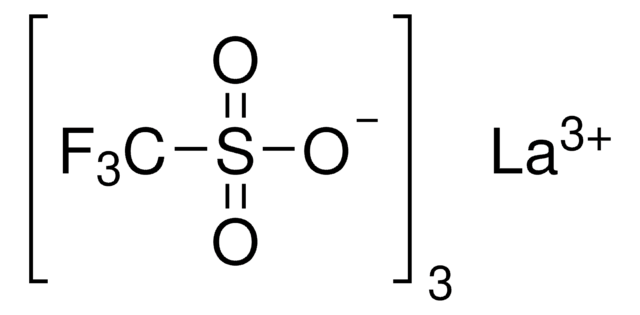
추천 제품
분석
98%
반응 적합성
core: dysprosium
reagent type: catalyst
reaction type: Ring-Opening Polymerization
SMILES string
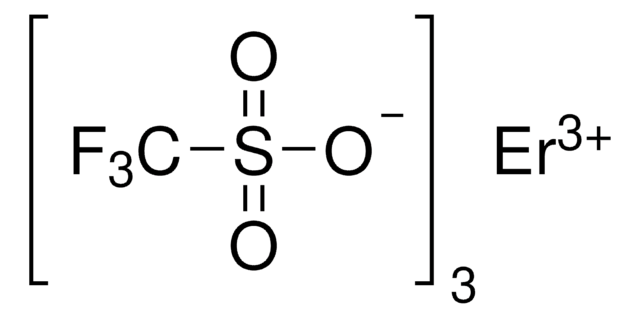
[Dy+3].[O-]S(=O)(=O)C(F)(F)F.[O-]S(=O)(=O)C(F)(F)F.[O-]S(=O)(=O)C(F)(F)F
InChI
1S/3CHF3O3S.Dy/c3*2-1(3,4)8(5,6)7;/h3*(H,5,6,7);/q;;;+3/p-3
InChI key
XSVCYDUEICANRJ-UHFFFAOYSA-K
일반 설명
애플리케이션
- Aza-Piancatelli rearrangement
- Friedel-Crafts alkylation
- Ring-opening polymerization reactions
- Microwave-assisted Kabachnik-Fields condensation
- Cycloaddition reactions (Lewis-acid catalyst)
- Fries rearrangement
- Enantioselective glyoxalate-ene reactions
- Aldol reaction of silyl enol ethers with aldehydes.
- As an effective catalyst for electrophilic substitution reactions of indoles with imines.
- As catalyst for the synthesis of 4-aminocyclopentenones and functionalized azaspirocycles, via intramolecular aza-Piancatelli rearrangement.
- As new curing initiator to study the curing of diglycidyl ether of bisphenol-A (DGEBA).
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
문서
The Fries rearrangement reaction is an organic name reaction which involves the conversion of phenolic esters into hydroxyaryl ketones on heating in the presence of a catalyst. Suitable catalysts for this reaction are Brønsted or Lewis acids such as HF, AlCl3, BF3, TiCl4, or SnCl4. The Fries rearrangement reaction is an ortho, para-selective reaction, and is used in the preparation of acyl phenols. This organic reaction has been named after German chemist Karl Theophil Fries.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.