417556
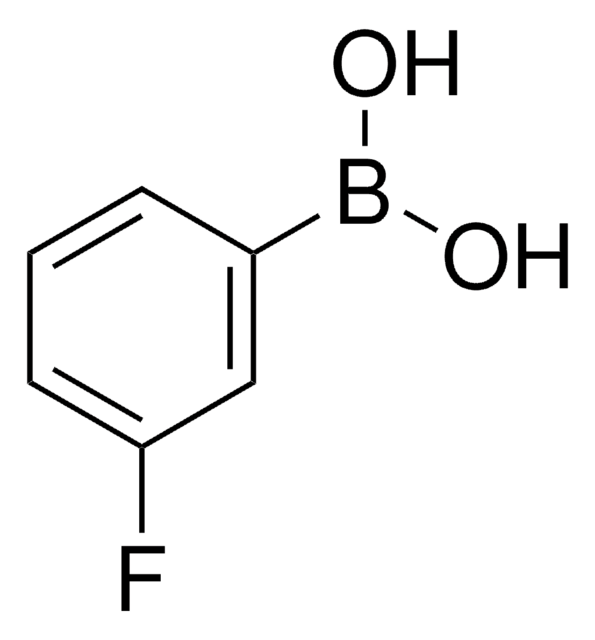
4-Fluorophenylboronic acid
≥95%
동의어(들):
(4-Fluorophenyl)boric acid, (4-Fluorophenyl)dihydroxyborane, (4-Fluorophenyl)dihydroxyboron, (p-Fluorophenyl)boric acid, 4-Fluorobenzeneboronic acid, p-Fluorobenzylboronic acid, p-Fluorophenylboronic acid, NSC 142683
로그인조직 및 계약 가격 보기
모든 사진(3)
About This Item
Linear Formula:
FC6H4B(OH)2
CAS Number:
Molecular Weight:
139.92
Beilstein:
2829653
MDL number:
UNSPSC 코드:
12352103
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
≥95%
양식
powder
mp
262-265 °C (lit.)
작용기
fluoro
SMILES string
OB(O)c1ccc(F)cc1
InChI
1S/C6H6BFO2/c8-6-3-1-5(2-4-6)7(9)10/h1-4,9-10H
InChI key
LBUNNMJLXWQQBY-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
4-Fluorophenylboronic acid can be used as a reactant in coupling reactions with arenediazonium tetrafluoroborates, iodonium salts, and iodanes. It is also used to make novel biologically active terphenyls.
It can also be used as a reactant in:
It can also be used as a reactant in:
- Suzuki coupling using microwave and triton B catalyst.
- Pd-catalyzed direct arylation of pyrazoles with phenylboronic acids.
- Mizoroki-Heck and Suzuki-Miyaura coupling reactions catalyzed by palladium nanoparticles.
- Cu-catalyzed Petasis reactions.
- Tandem-type Pd(II)-catalyzed oxidative Heck reaction and intramolecular C-H amidation sequence.
- Ruthenium catalyzed direct arylation.
- Rh-catalyzed asymmetric conjugate additions.
- Ligand-free copper-catalyzed coupling of nitro arenes with arylboronic acids.
- Regioselective arylation and alkynylation by Suzuki-Miyaura and Sonogashira cross-coupling reactions.
- Suzuki cross-coupling of tetrabromothiophene.
- Palladium-catalyzed addition to nitriles.
기타 정보
Contains varying amounts of anhydride
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
Tetrahedron Letters, 48, 845-845 (2007)
Tetrahedron Letters, 37, 3857-3857 (1996)
Yuvraj Satkar et al.
Frontiers in chemistry, 8, 563470-563470 (2020-11-17)
The chemoselective reaction of the C- followed by the O-centered naphthyl radicals with the more electron-deficient hypervalent bond of the diaryliodonium(III) salts is described. This discovered reactivity constitutes a new activation mode of the diaryliodonium(III) salts which enabled a one-pot
Microwave-enhanced triton B catalyzed Suzuki coupling reaction
Meshram, H. M.; et al.
Indian J. Chem. B, 51, 362-365 (2012)
J J Li et al.
Journal of medicinal chemistry, 39(9), 1846-1856 (1996-04-26)
A novel series of terphenyl methyl sulfones and sulfonamides have been shown to be highly potent and selective cyclooxygenase-2 (COX-2) inhibitors. The sulfonamide analogs 17 and 21 were found to be much more potent COX-2 inhibitors and orally active anti-inflammatory
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.