377953
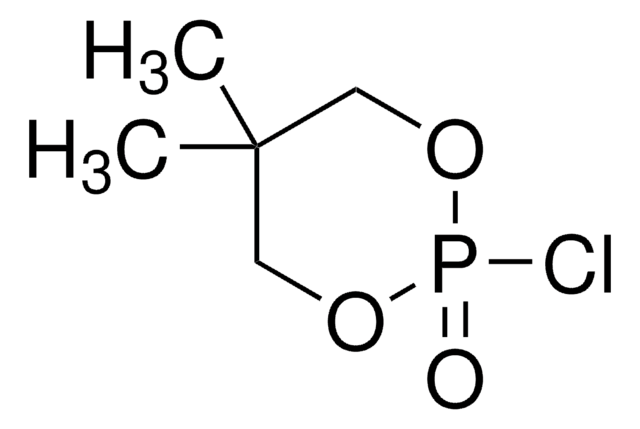
2-Chloro-1,3,2-dioxaphospholane 2-oxide
동의어(들):
2-Chloro-2-oxo-1,3,2-dioxaphospholane, Ethylene glycol chlorophosphate
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
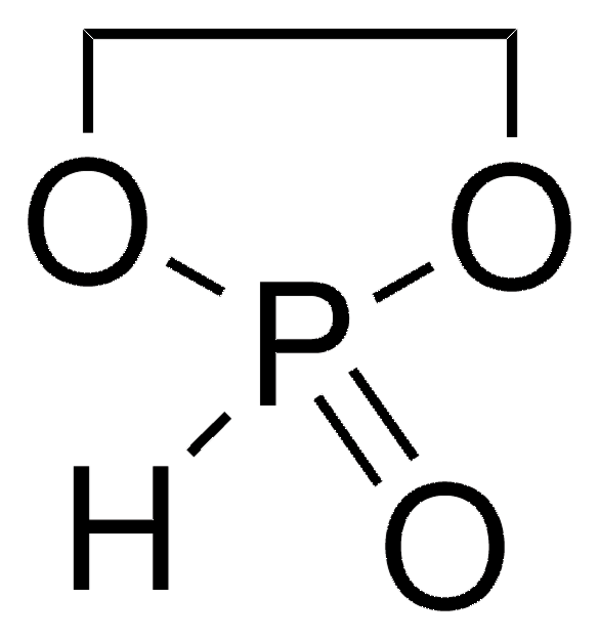
실험식(Hill 표기법):
C2H4ClO3P
CAS Number:
Molecular Weight:
142.48
Beilstein:
606582
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
양식
liquid
Quality Level
불순물
<10% 2-Chloro-1,3,2-dioxaphospholane
refractive index
n20/D 1.45 (lit.)
bp
89-91 °C/0.8 mmHg (lit.)
mp
12-14 °C (neat) (lit.)
density
1.55 g/mL at 25 °C (lit.)
저장 온도
−20°C
SMILES string
ClP1(=O)OCCO1
InChI
1S/C2H4ClO3P/c3-7(4)5-1-2-6-7/h1-2H2
InChI key
SBMUNILHNJLMBF-UHFFFAOYSA-N
일반 설명
2-Chloro-1,3,2-dioxaphospholane 2-oxide (COP) is a cyclic chlorophosphate reagent that can be prepared from 2-chloro-1,3,2-dioxaphospholane by reacting with molecular oxygen .
2-Chloro-1,3,2-dioxaphospholane 2-oxide is used in esterification reactions for cyclic phosphate synthesis, also reacts with phenyl grignard reagents.
2-Chloro-1,3,2-dioxaphospholane 2-oxide is used in esterification reactions for cyclic phosphate synthesis, also reacts with phenyl grignard reagents.
애플리케이션
2-Chloro-1,3,2-dioxaphospholane 2-oxide may be used in the synthesis of:
- 2-methacryloyloxyethylphosphorylcholine
- miltefosine (hexadecylphosphocholine, MT) analogs
- phosphoric acid 2-trimethylamino-ethyl ester undec-10-enyl ester
- uridine nucleolipid, (2′,3′-O-16-hentriacontanyliden-uridine-5′-phosphocholine, PUPC)
- adenosine nucleoamphiphile, (2′,3′-O-16-hentriacontanyliden-adenosine-5′-phosphocholine, PAPC)
- structurally related phospholipids which are either conformationally restricted or flexible
- phosphatidylcholines
Reactant for:
- Synthesis of amino-functionalized hybrid hydrocarbon/fluorocarbon double-chain phospholipid
- Synthesis of UV-polymerizable lipids via Chabrier reaction
- Syntheses of block copolymers of poly(aliphatic ester) with clickable polyphosphoester
- Imprinting molecular recognition sites on multiwalled carbon nanotubes surface for electrochemical detection of insulin in real samples
- Synthesis of a zwitterionic silane
- Synthesis of a core-shell-corona micelle stabilized by reversible cross-linkage for intracellular drug delivery
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
보충제 위험성
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point (°F)
>235.4 °F - closed cup
Flash Point (°C)
> 113 °C - closed cup
개인 보호 장비
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
이미 열람한 고객
Toward the Efficient Synthesis of New Phosphopantothenate Derivatives by Using Chlorophosphate Reagents.
Pahor J, et al.
Synthesis, 48(21), 3763-3772 (2016)
Biomimetic honeycomb-structured surfaces formed from block copolymers incorporating acryloyl phosphorylcholine.
Stenzel MH and Davis TP.
Australian Journal of Chemistry, 56(10), 1035-1038 (2003)
Xue Jiang et al.
Nanomaterials (Basel, Switzerland), 9(2) (2019-02-16)
An efficient strategy for growing thermo-sensitive polymers from the surface of exfoliated graphene oxide (GO) is reported in this article. GO sheets with hydroxyls and epoxy groups on the surface were first prepared by modified Hummer's method. Epoxy groups on
Louis Moreau et al.
Journal of the American Chemical Society, 130(44), 14454-14455 (2008-10-15)
Supramolecular assembly formation resulting from molecular recognition between complementary nucleolipids has been visualized in real time at the micrometer scale.
Rong Yang et al.
Journal of controlled release : official journal of the Controlled Release Society, 289, 94-101 (2018-06-23)
Chemical permeation enhancers (CPEs) can enable antibiotic flux across the tympanic membrane. Here we study whether combinations of CPEs (sodium dodecyl sulfate, limonene, and bupivacaine hydrochloride) are synergistic and whether they could increase the peak drug flux. Synergy is studied
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.