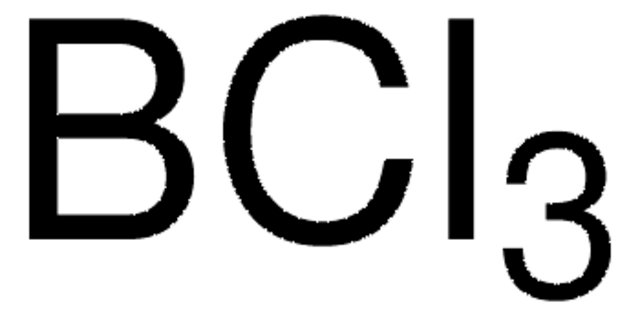추천 제품
양식
liquid
Quality Level
반응 적합성
core: boron
reagent type: Lewis acid
reagent type: catalyst
농도
1.0 M in heptane
density
0.74 g/mL at 25 °C
저장 온도
2-8°C
SMILES string
ClB(Cl)Cl
InChI
1S/BCl3/c2-1(3)4
InChI key
FAQYAMRNWDIXMY-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
- Boron trichloride, a Lewis acid, is a general reagent for the cleavage of a wide range of ether and acetal protecting groups.
- It is a useful reagent to directly convert aromatic aldehydes to the corresponding gem-dichlorides.
- It can also be used in the transmetallation reaction with less nucleophilic reagents such as tin and zirconium organometallic compounds to synthesize a variety of organoboranes.
For preparing methyl esters of fatty acids and for transesterification of triglycerides.
포장
The 25 mL Sure/Seal™ bottle is recommended as a single-use bottle. Repeated punctures will likely result in decreased performance of product.
법적 정보
Sure/Seal is a trademark of Sigma-Aldrich Co. LLC
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 2 Oral - Acute Tox. 3 Inhalation - Aquatic Acute 1 - Aquatic Chronic 1 - Asp. Tox. 1 - Eye Dam. 1 - Flam. Liq. 2 - Repr. 1B - Skin Corr. 1B - STOT SE 3
표적 기관
Respiratory system
보충제 위험성
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point (°F)
35.6 °F - closed cup
Flash Point (°C)
2 °C - closed cup
개인 보호 장비
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
이미 열람한 고객
Impact of Different Environmental Stimuli on the Release of 1-MCP from Boron-MCP Complexes.
Shahrin T, et al.
Journal of Plant Studies, 6(1), 46-46 (2016)
Boron Trichloride
Miyaura N, et al.
e-EROS Encyclopedia of Reagents for Organic Synthesis (2006)
Migration of 1-alkenyl groups from zirconium to boron compounds.
Cole T E, et al.
Organometallics, 10(10), 3777-3781 (1991)
Conversion of aromatic aldehydes to gem-dichlorides using boron trichloride. A new highly efficient method for preparing dichloroarylmethanes.
Kabalka G W and Wu Z
Tetrahedron Letters, 41(5), 579-581 (2000)
Guillaume Reinisch et al.
The journal of physical chemistry. A, 115(18), 4786-4797 (2011-04-16)
We report on a theoretical study of the gas-phase decomposition of boron trichloride in the presence of hydrogen radicals using ab initio energetic calculations coupled to TST, RRKM, and VTST-VRC kinetic calculations. In particular, we present an addition-elimination mechanism (BCl(3)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.