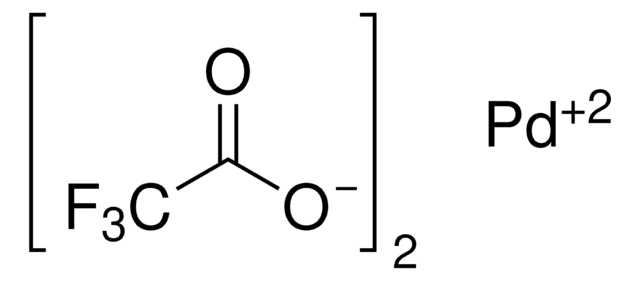
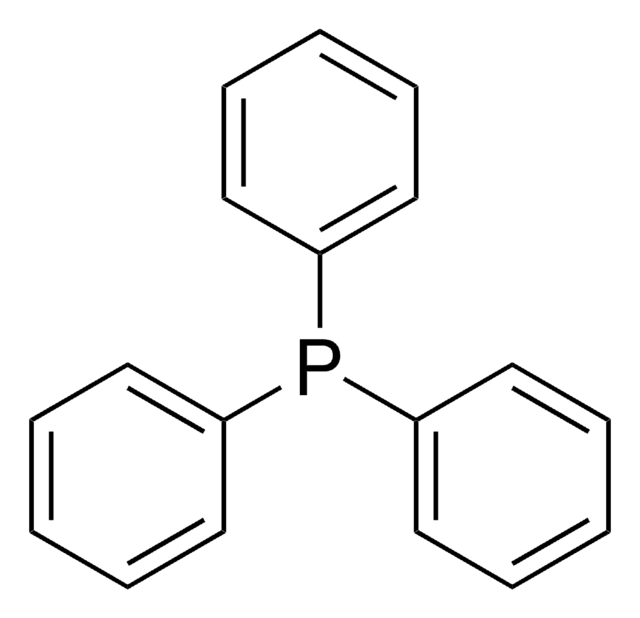
추천 제품
Quality Level
분석
99.995%
양식
powder
반응 적합성
core: palladium
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
mp
678-680 °C (lit.)
density
4 g/mL at 25 °C (lit.)
SMILES string
Cl[Pd]Cl
InChI
1S/2ClH.Pd/h2*1H;/q;;+2/p-2
InChI key
PIBWKRNGBLPSSY-UHFFFAOYSA-L
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Palladium(II) chloride is used as a precursor to prepare palladium catalysts for various reactions like Heck coupling, cascade reaction, Buchward-Hartwig coupling. It is also used as an oxidizing agent.
애플리케이션
Application Guide for Palladium Catalyzed Cross-Coupling Reactions
Used in the synthesis of semiconducting metal-containing polymers in which the polypyrrole backbone has a conformational energy minimum and is nearly planar.
Used in the synthesis of semiconducting metal-containing polymers in which the polypyrrole backbone has a conformational energy minimum and is nearly planar.
Palladium(II) chloride can be used as a catalyst in:
- Carbonylation of ketones to yield diesters.
- Homo-coupling of aryl bromides using ascorbic acid and EDTA..
- Acetylation of alcohols with vinyl acetate.
- Arylation of 2-furaldehyde to yield 5-aryl-2-formylfuran derivatives.
Pd precursor used as an oxidizing agent and as a source of Pd(0) complexes, e.g. Heck coupling, Cascade reaction, Buchward-Hartwig coupling.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Dam. 1 - Met. Corr. 1 - Skin Sens. 1
Storage Class Code
8B - Non-combustible corrosive hazardous materials
WGK
WGK 3
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
이미 열람한 고객
Palladium (II) chloride/EDTA-catalyzed biaryl homo-coupling of aryl halides in aqueous medium in the presence of ascorbic acid
Ram RN and Singh V
Tetrahedron Letters, 47(43), 7625-7628 (2006)
Mihai S Viciu et al.
Organic letters, 5(9), 1479-1482 (2003-04-26)
Palladacycle dimers possessing bridging halides can be easily cleaved by using N-heterocyclic carbenes (NHCs) to generate novel monomeric complexes. The structure of one of these was determined by single-crystal diffraction study and consists of a square-planar coordination around the palladium
Regioselective palladium-catalyzed arylation of 2-furaldehyde
McClure MS, et al.
Organic Letters, 3(11), 1677-1680 (2001)
A new generation of air stable, highly active Pd complexes for C--C and C--N coupling reactions with aryl chlorides.
Anita Schnyder et al.
Angewandte Chemie (International ed. in English), 41(19), 3668-3671 (2002-10-09)
O Hamed et al.
The Journal of organic chemistry, 66(1), 180-185 (2001-06-30)
Unsubstituted or alkyl-substituted cyclic ketones react with PdCl2 in methanol under a CO atmosphere to give mainly acyclic diesters along with some acyclic chloro-substituted monoesters. The monosubstituted cyclic ketones, 2-hydroxy- and 2-methoxycyclohexanone, do not give ring cleavage but rather produce
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.