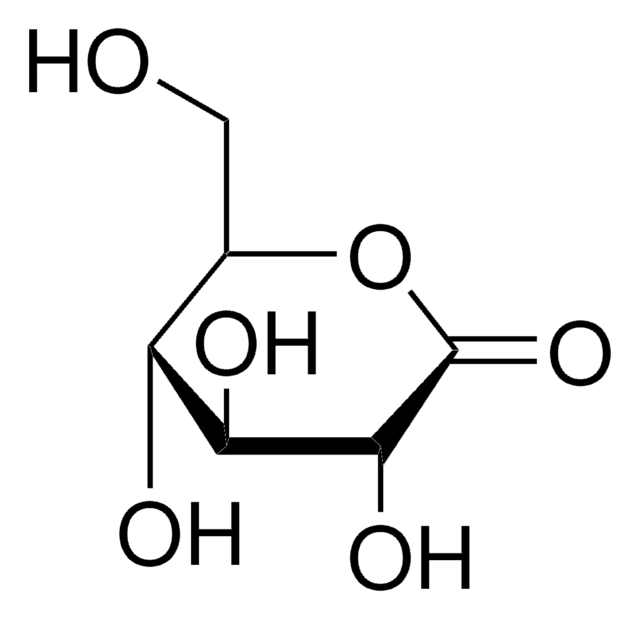
추천 제품
Quality Level
분석
95%
양식
solid
광학 활성
[α]19/D +55°, c = 4 in H2O
mp
187-190 °C (lit.)
저장 온도
2-8°C
SMILES string
OC[C@H](O)[C@H]1OC(=O)[C@@H](O)[C@H]1O
InChI
1S/C6H10O6/c7-1-2(8)5-3(9)4(10)6(11)12-5/h2-5,7-10H,1H2/t2-,3+,4-,5+/m0/s1
InChI key
SXZYCXMUPBBULW-SKNVOMKLSA-N
애플리케이션
- Development and validation of an analysis method: Discusses the use of L-gulonic acid γ-lactone as a matrix effect inhibitor in the validation of a method for pesticide residues by gas chromatography–tandem mass spectrometry (Saegusa, Nomura, Takao, Hamaguchi, 2021).
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
Beata A Wolucka et al.
The FEBS journal, 273(19), 4435-4445 (2006-09-08)
The last step of the biosynthesis of L-ascorbic acid (vitamin C) in plants and animals is catalyzed by L-gulono-1,4-lactone oxidoreductases, which use both L-gulono-1,4-lactone and L-galactono-1,4-lactone as substrates. L-gulono-1,4-lactone oxidase is missing in scurvy-prone, vitamin C-deficient animals, such as humans
Marjan Jeselnik et al.
Organic letters, 5(15), 2651-2653 (2003-07-19)
[reaction: see text] A new synthesis of L-noviose (11), a sugar moiety of novobiocin, is presented. D-Gulonolactone was initially converted in a few steps to the key ester derivative 7 [1-O-benzyl methyl 2,3-O-(1-methylethylidene)-alpha-L-lyxofuranosiduronate]. An appropriate selection of protecting groups enabled
G W Fleet et al.
Carbohydrate research, 205, 269-282 (1990-09-19)
The synthesis of the enantiomers of 6-epicastanospermine and of 1,6-diepicastanospermine from the enantiomeric gulonolactones is reported and the structure of the former is established as (1S,6R,7R,8R,8aR)-1,6,7,8-tetrahydroxyoctahydroindolizine. The inhibitory activities of the diastereomers against the amyloglucosidase-catalysed hydrolysis of p-nitrophenyl alpha-D-glucopyranoside were
F Puskás et al.
FEBS letters, 430(3), 293-296 (1998-08-04)
The orientation of gulonolactone oxidase activity was investigated in rat liver microsomes. Ascorbate formation upon gulonolactone addition resulted in higher intravesicular than extravesicular ascorbate concentrations in native microsomal vesicles. The intraluminal ascorbate accumulation could be prevented or the accumulated ascorbate
A Krasnov et al.
Genetic analysis : biomolecular engineering, 15(3-5), 115-119 (1999-12-22)
The reviewed studies addressed the possibility of using gene transfer for correction of L-ascorbic acid biosynthesis and carbohydrate utilization in rainbow trout. Analyses of enzymatic activities in the L-AAB pathway indicated that reasons for the lack of L-AA production can
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.