추천 제품
분석
99%
refractive index
n20/D 1.501 (lit.)
bp
210-211 °C (lit.)
density
1.047 g/mL at 25 °C (lit.)
작용기
ester
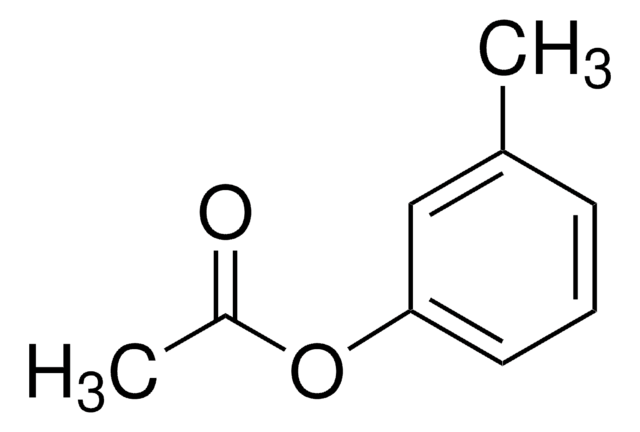
SMILES string
CC(=O)Oc1ccc(C)cc1
InChI
1S/C9H10O2/c1-7-3-5-9(6-4-7)11-8(2)10/h3-6H,1-2H3
InChI key
CDJJKTLOZJAGIZ-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
애플리케이션
신호어
Warning
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point (°F)
194.0 °F - closed cup
Flash Point (°C)
90 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
문서
The Fries rearrangement reaction is an organic name reaction which involves the conversion of phenolic esters into hydroxyaryl ketones on heating in the presence of a catalyst. Suitable catalysts for this reaction are Brønsted or Lewis acids such as HF, AlCl3, BF3, TiCl4, or SnCl4. The Fries rearrangement reaction is an ortho, para-selective reaction, and is used in the preparation of acyl phenols. This organic reaction has been named after German chemist Karl Theophil Fries.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.