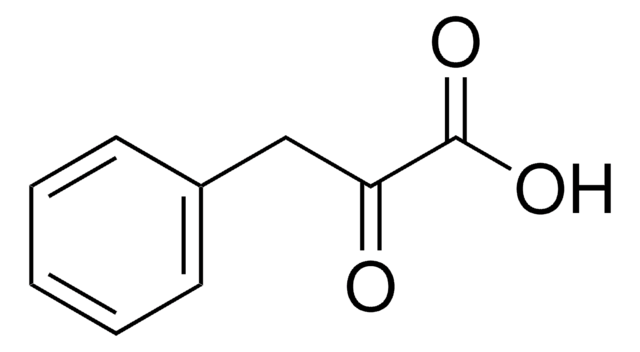
281492
Ethyl benzoylacetate
technical grade, 90%
동의어(들):
Benzoylacetic acid ethyl ester, Ethyl 3-oxo-3-phenylpropionate
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
Linear Formula:
C6H5COCH2COOC2H5
CAS Number:
Molecular Weight:
192.21
Beilstein:
389944
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Grade
technical grade
Quality Level
vapor density
6.6 (vs air)
분석
90%
양식
liquid
refractive index
n20/D 1.52 (lit.)
bp
265-270 °C (lit.)
solubility
alcohol: miscible
diethyl ether: miscible
water: insoluble
density
1.11 g/mL at 25 °C (lit.)
작용기
ester
ketone
phenyl
SMILES string
CCOC(=O)CC(=O)c1ccccc1
InChI
1S/C11H12O3/c1-2-14-11(13)8-10(12)9-6-4-3-5-7-9/h3-7H,2,8H2,1H3
InChI key
GKKZMYDNDDMXSE-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Ethyl benzoylacetate is an ester. It undergoes microbial reduction by bakers′ yeast (Saccharomyces cerevisiae), Beauveria sulfurescens or Geotrichum candidum to afford ethyl (S)-3-hydroxy-3-phenylpropionate. It undergoes Claisen condensation reaction with malononitrile to afford 2-cyano-5-phenyl-3,5-dioxopentanonitrile which on cyclization followed by coupling with diazonium salts yields azo derivatives.
애플리케이션
Ethyl benzoylacetate was sed in the preparation of:
- triazipinones
- ethyl 2-fluoro-2-benzolyacetate
- 1,2,4-triazolo[1,5-a]pyrimidine antibiotic essramycin
- benzo[c]furan-4,7-diones
- iodonium ylides
포장
Packaged in glass bottles
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point (°F)
284.0 °F - closed cup
Flash Point (°C)
140 °C - closed cup
개인 보호 장비
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
이미 열람한 고객
Ugo Battaglia et al.
Journal of natural products, 73(11), 1938-1939 (2010-09-15)
A short synthesis of the 1,2,4-triazolo[1,5-a]pyrimidine antibiotic essramycin is described involving condensation of aminoguanidine with ethyl benzoylacetate to give an amino-1,2,4-triazole, followed by condensation with ethyl acetoacetate to form the pyrimidone ring.
Asymmetric synthesis of both enantiomers of fluoxetine via microbiological reduction of ethyl benzoylacetate.
Chenevert R, et al.
Tetrahedron, 48(33), 6769-6776 (1992)
Mohamed G Badrey et al.
Molecules (Basel, Switzerland), 17(10), 11538-11553 (2012-09-29)
A number of interesting heterocycles were prepared through interaction of the intermediate 3-amino-8-hydroxy-4-imino-6-methyl-5-phenyl-4,5-dihydro-3H-chromeno-[2,3-d]pyrimidine (1) and reagents such as hydrazonyl halides 2 to furnish triazine derivatives 4a-l. Reaction of 1 with phenacyl bromide afforded compound 5. Moreover, the title compound 1
Kuang-Po Chen et al.
Organic & biomolecular chemistry, 7(19), 4074-4081 (2009-09-19)
A manganese(III)-mediated reaction between 2-benzoyl-1,4-benzoquinones and 1,3-dicarbonyl compounds that produces benzo[c]furan-4,7-diones and anthracene-1,4-diones with high chemoselectivity is described. With ethyl butyrylacetate, by changing the solvent, benzo[c]furan-4,7-diones and anthracene-1,4-diones can be generated in high chemoselectivities. With ethyl benzoylacetate, N ,N-dimethyl acetoacetamide
The reaction of ethyl benzoylacetate with malononitrile: a novel synthesis of some pyridazine, pyridazino [2, 3-a] quinazoline and pyrrole derivatives.
Abdelrazek FM, et al.
Tetrahedron, 57(9), 1813-1817 (2001)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.