추천 제품
제품명
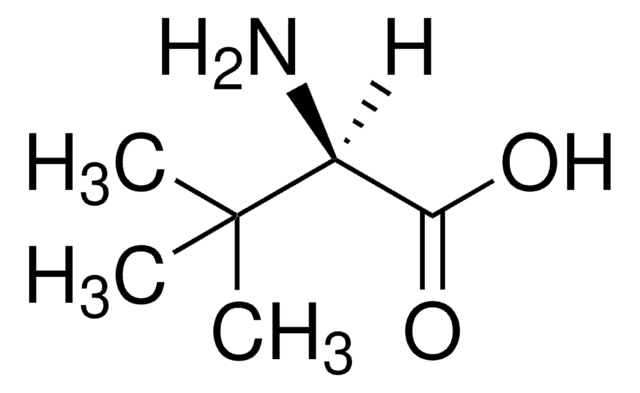
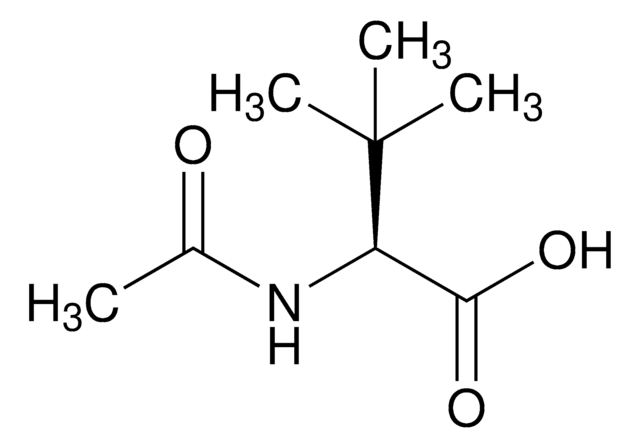
L-tert-Leucine, 99%
Quality Level
분석
99%
양식
powder
광학 활성
[α]20/D −9.5°, c = 3 in H2O
광학 순도
ee: 99% (GLC)
반응 적합성
reaction type: solution phase peptide synthesis
mp
≥300 °C (lit.)
응용 분야
peptide synthesis
SMILES string
CC(C)(C)[C@H](N)C(O)=O
InChI
1S/C6H13NO2/c1-6(2,3)4(7)5(8)9/h4H,7H2,1-3H3,(H,8,9)/t4-/m1/s1
InChI key
NPDBDJFLKKQMCM-SCSAIBSYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
L-tert-Leucine is an amino acid used as a precursor for synthesizing chiral tridentate Schiff base ligands.
애플리케이션
L-tert-Leucine can be used:
- As a key precursor in the synthesis of a chiral phosphinooxazoline ligand, (S)-tert-butylPHOX.
- In the synthesis of chiral copper(II) polymers that can catalyze the kinetic resolution of secondary alcohols by acylation.
- In metal-free tandem radical cyclization reactions to synthesize 6-alkyl/acyl phenanthridines.
- In the preparation of tert-leucine-derived N-acetylthiazolidinethione auxiliary that provides high levels of diastereoselection in acetate aldol reactions with a variety of aldehydes.
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
Use of unprotected amino acids in metal-free tandem radical cyclization reactions: divergent synthesis of 6-alkyl/acyl phenanthridines.
Lu S C, et al.
Royal Society of Chemistry Advances, 7(88), 55891-55896 (2017)
Highly Selective Asymmetric Acetate Aldol Reactions of an N-Acetyl Thiazolidinethione Reagent.
Zhang Y, et al.
Organic Letters, 6(1), 23-25 (2004)
Synthesis, structure and application of chiral copper (II) coordination polymers for asymmetric acylation.
Jammi S, et al.
Inorganic Chemistry, 47(12), 5093-5098 (2008)
Preparation of (S)?tert?Butyl PHOX: (Oxazole, 4?(1, 1?Dimethylethyl)?2?[2?(Diphenylphosphino) Phenyl]?4, 5?Dihydro?(4S)?).
Krout M R, et al.
Organic Syntheses, 86, 181-193 (2003)
Philippe Bisel et al.
Organic & biomolecular chemistry, 6(15), 2655-2665 (2008-07-18)
The unique reactivity pattern elicited by the crowded tert-butyl group is highlighted by summarising characteristic applications. Starting from the use of this simple hydrocarbon moiety in chemical transformations, via its relevance in Nature and its implication in biosynthetic and biodegradation
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.