모든 사진(1)
About This Item
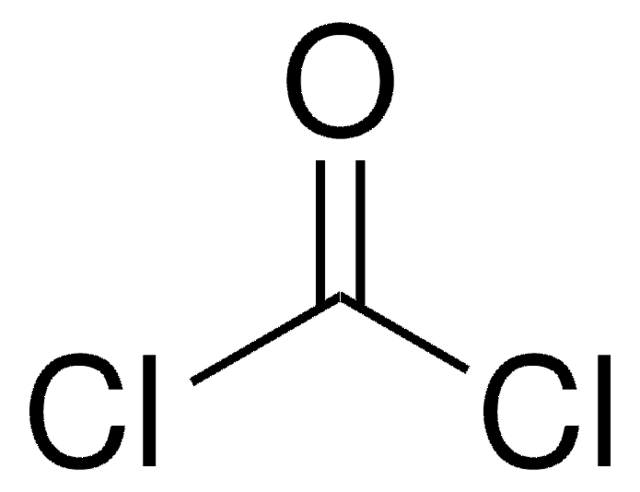
Linear Formula:
ClCOOCCl3
CAS Number:
Molecular Weight:
197.83
Beilstein:
970225
EC Number:
MDL number:
UNSPSC 코드:
12352108
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
≥97.0% (GC)
refractive index
n20/D 1.458
bp
20 °C/10 mmHg (lit.)
density
1.639 g/mL at 20 °C
작용기
chloro
저장 온도
2-8°C
SMILES string
ClC(=O)OC(Cl)(Cl)Cl
InChI
1S/C2Cl4O2/c3-1(7)8-2(4,5)6
InChI key
HCUYBXPSSCRKRF-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
Reactant for preparation of:
- Cyclic carbamimidates using a monophosphine gold(i) catalyst
- N-Alkenyl and cycloalkyl carbamates as dual acting histamine H3 and H4 receptor ligands
- Prostate-specific membrane antigen-targeted anticancer prodrugs
- Potential west nile virus protease inhibitors
- Antibody-drug conjugates (ADCs)
- Erythromycin A derivatives
Trichloromethyl chloroformate (TCF) is an effective alternative to phosgene. It can react with amines, amino acids and amino alcohols to give the corresponding isocyanates, isocyanato acid chlorides and isocyanato chloroformates.
TCF can also be used:
TCF can also be used:
- To synthesize N-carboxy α-amino acid anhydrides.
- As an acylating agent to synthesize oxazolidinones from α-amino alcohols.
- As a dehydrating agent to synthesize aromatic diisocyanides in the presence of triethylamine.
기타 정보
Easy to handle substitute for phosgene; In-situ charcoal-catalyzed decomposition to phosgene and reaction with amino acids to N-carboxy anhydrides
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 2 Inhalation - Acute Tox. 4 Oral - Skin Corr. 1B
Storage Class Code
6.1B - Non-combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
이미 열람한 고객
Topics in Inorganic and General Chemistry, 24, 535-541 (1996)
L.N. Pridgen et al.
The Journal of Organic Chemistry, 54, 3231-3231 (1989)
Trichloromethyl chloroformate. Reaction with amines, amino acids, and amino alcohols.
Kurita K, et al.
The Journal of Organic Chemistry, 41(11), 2070-2071 (1976)
H. Ogura et al.
Bulletin of the Chemical Society of Japan, 56, 2485-2485 (1983)
Single-pot reductive conversion of amino acids to their respective 2-oxazolidinones employing trichloromethyl chloroformate as the acylating agent: A multigram synthesis.
Pridgen L N, et al.
The Journal of Organic Chemistry, 54(13), 3231-3233 (1989)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.