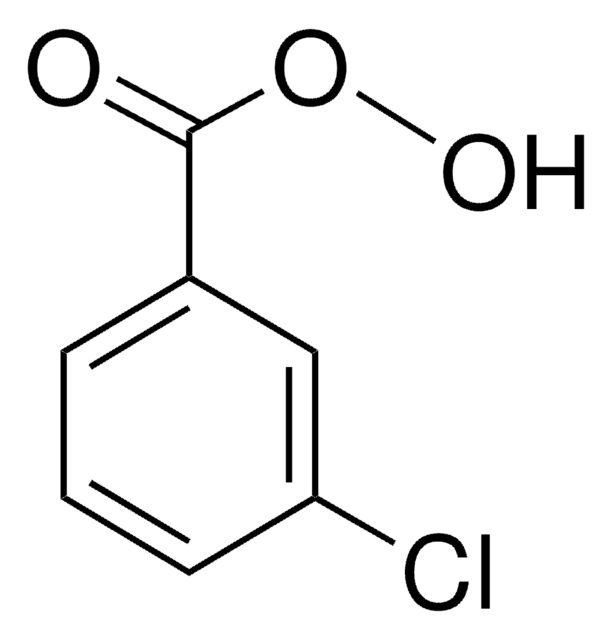
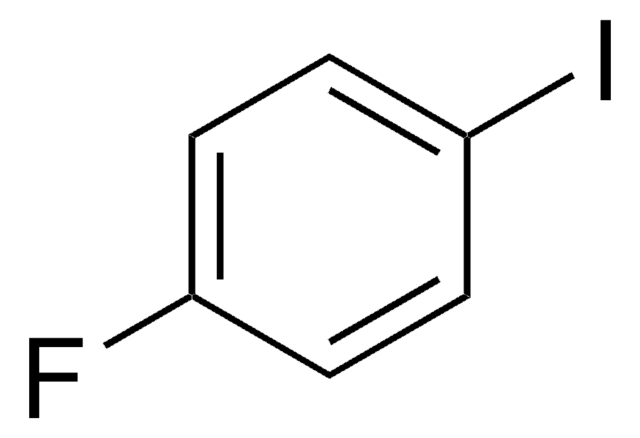
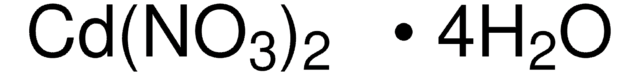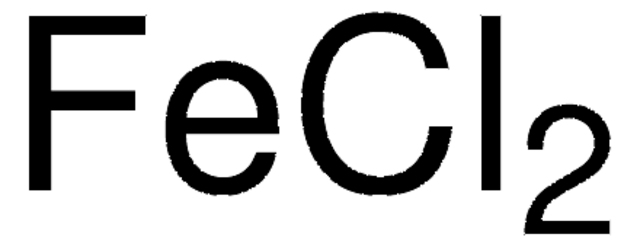추천 제품
양식
powder
Quality Level
반응 적합성
reagent type: oxidant
저장 온도
2-8°C
SMILES string
[K+].[K+].[O]N(S([O-])(=O)=O)S([O-])(=O)=O
InChI
1S/2K.H2NO7S2/c;;2-1(9(3,4)5)10(6,7)8/h;;(H,3,4,5)(H,6,7,8)/q2*+1;/p-2
InChI key
IHSLHAZEJBXKMN-UHFFFAOYSA-L
일반 설명
Potassium nitrosodisulfonate is an oxidizing reagent that can be used to convert phenols, naphthols, and anilines to quinones, benzylic alcohols to aldehydes or ketones, and amino acids to α-keto acids. It can also be used for the preparation of heterocyclic quinones and oxidative aromatization.
애플리케이션
Potassium nitrosodisulfonate can be used as an oxidizing reagent for the oxidation of:
- Aromatic amines to their corresponding quinones.
- Hydroethidine to 2-hydroxyethidium.
- Tyrosine to o-quinones.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Water-react 1
보충제 위험성
Storage Class Code
4.3 - Hazardous materials which set free flammable gases upon contact with water
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
Z L Liu et al.
Chemistry and physics of lipids, 56(1), 73-80 (1990-11-01)
The reaction between Fremy's salt and alpha-tocopherol (VE), ascorbic acid (VC) and its lipophilic derivatives ascorbyl-6-caprylate (VC-8), 6-laurate (VC-12) and 6-palmitate (VC-16) were studied by stopped-flow ESR spectroscopy in cetyl trimethylammonium bromide (CTAB) and sodium dodecyl sulfate (SDS) micelles, as
J. Wood Chem. Technol., 9, 491-491 (1989)
I Islam et al.
Journal of medicinal chemistry, 34(10), 2954-2961 (1991-10-01)
Described herein are structure-activity studies of new antitumor agents based on the pyrrolo[1,2-a]benzimidazole (PBI) ring system. These compounds were designed as new DNA cross-linkers mimicking the mitomycin antitumor agents. Actually, the PBI derivatives were found to have anthracycline-like features: (i)
S Pötsch et al.
FEBS letters, 374(1), 95-99 (1995-10-23)
The rate of reduction of the tyrosyl radical in the small subunit of ribonucleotide reductase (protein R2) from E. coli, mouse, and herpes simplex virus (HSV-2) by a series of p-alkoxyphenols with different alkyl chains, have been studied by stopped-flow
F J Hornicek et al.
Chemico-biological interactions, 55(3), 289-302 (1985-11-01)
Nitroxyldisulfonate [Fremy's salt; (KSO3)2NO.] and bisulfite (NaHSO3) have abolished periodic acid (H5IO6)-induced blastogenesis of human peripheral blood lymphocytes (HPBL), but only inhibited the blastogenic response of H5IO6-oxidized rat and mouse lymphocytes, as determined by the rates of nucleic acids synthesis
문서
Oxidation and reduction reactions are some of the most common transformations encountered in organic synthesis
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.