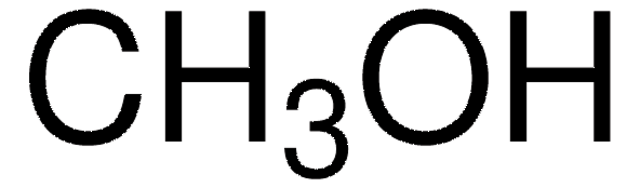추천 제품
양식
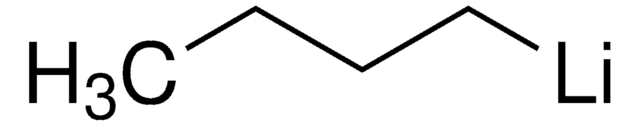
liquid
Quality Level
반응 적합성
reagent type: reductant
농도
1.0 M in methylene chloride
density
1.23 g/mL at 25 °C
저장 온도
2-8°C
SMILES string
CC(C)C[AlH]CC(C)C
InChI
1S/2C4H9.Al.H/c2*1-4(2)3;;/h2*4H,1H2,2-3H3;;
InChI key
AZWXAPCAJCYGIA-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Diisobutylaluminum hydride is a reducing agent for many functional groups commonly used in organic synthesis.
애플리케이션
Diisobutylaluminum hydride solution (1 M in dichloromethane) has been used in the conversion of esters into α-fluoro-α,β-unsaturated esters in the presence of fluorocarboalkoxy-substituted dialkyl phosphonate anions via Horner-Wadsworth-Emmons reaction. It can be used in the multi-step synthesis of (S)-2-methyl tetrahydropyridine-N-oxide, a key structural moiety of bio-active compounds like himbacine and solenopsin-A.
Used in Pd-catalyzed reductive debromination of secondary alkyl bromides. O-Debenzylation and ring opening of perbenzylated furanosides. Convenient in situ generation of HZrCp2Cl from ZrCp2Cl2 and DIBAL-H.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Carc. 2 - Eye Dam. 1 - Pyr. Liq. 1 - Skin Corr. 1B - STOT SE 3 - Water-react 1
표적 기관
Central nervous system
보충제 위험성
Storage Class Code
4.2 - Pyrophoric and self-heating hazardous materials
WGK
WGK 2
이미 열람한 고객
An enantioselective route to trans-2, 6-disubstituted piperidines
Chackalamannil S
Tetrahedron, 53(32), 11203-11210 (1997)
Reduction-olefination of esters: a new and efficient synthesis of. alpha.-fluoro. alpha.,. beta.-unsaturated esters.
Thenappan A
The Journal of Organic Chemistry, 55(15), 4639-4642 (1990)
Diisobutylaluminum Hydride.
Galatsis P
e-EROS Encyclopedia of Reagents for Organic Synthesis (2008)
Reaction of diisobutylaluminum hydride with selected organic compounds containing representative functional groups
Yoon N M
The Journal of Organic Chemistry, 50(14), 2443-2450 (1985)
Damien Webb et al.
Organic letters, 14(2), 568-571 (2011-12-31)
A continuous flow system for the multiparameter (flow rate, temperature, residence time, stoichiometry) optimization of the DIBALH reduction of esters to aldehydes is described. Incorporating an in-line quench (MeOH), these transformations are generally complete in fewer than 60 s. Mixing
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.