210145
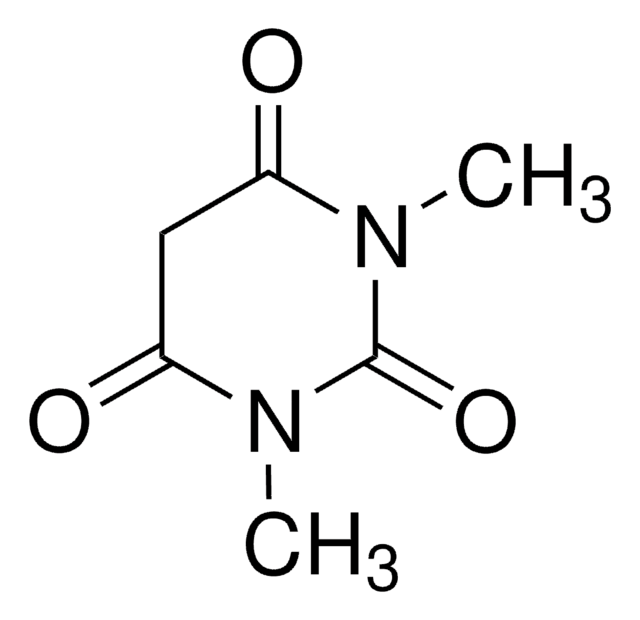
2,2-Dimethyl-1,3-dioxane-4,6-dione
98%
동의어(들):
Malonic acid cyclic isopropylidene ester, Meldrum’s acid, cycl-Isopropylidene malonate
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C6H8O4
CAS Number:
Molecular Weight:
144.13
Beilstein:
117310
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
98%
양식
solid
mp
92-96 °C (lit.)
solubility
dioxane: soluble 5%, clear to very slightly hazy, colorless to faintly yellow
작용기
ester
ketal
저장 온도
2-8°C
SMILES string
CC1(C)OC(=O)CC(=O)O1
InChI
1S/C6H8O4/c1-6(2)9-4(7)3-5(8)10-6/h3H2,1-2H3
InChI key
GXHFUVWIGNLZSC-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
2,2-Dimethyl-1,3-dioxane-4,6-dione (Meldrum′s acid) is widely used in organic synthesis, especially for multiple C-C bond formations due to its adequate acidity (pKa 4.83) and steric rigidity. Knoevenagel condensation reaction between aldehydes and Meldrum′s acid are accelerated in ionic liquids.
Meldrum′s acid is used as a valuable starting material to synthesize heterocycles and as intermediates in organic synthesis reactions.
Meldrum′s acid is used as a valuable starting material to synthesize heterocycles and as intermediates in organic synthesis reactions.
애플리케이션
2,2-Dimethyl-1,3-dioxane-4,6-dione was used in the synthesis of:
- macrocyclic β-keto lactone
- 4-pyridyl-substituted heterocycles
- 2-substituted indoles
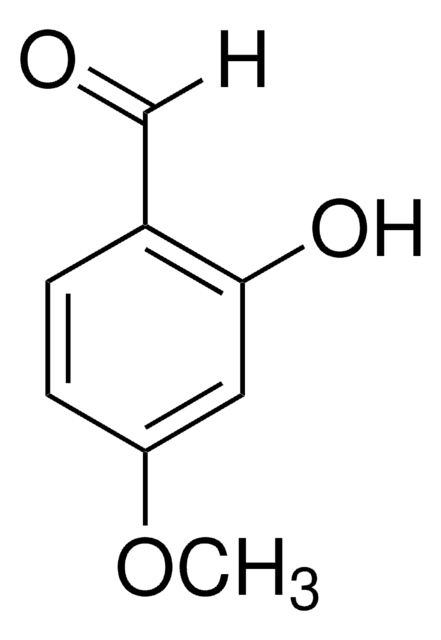
- isofraxidin.
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
Aaron M Dumas et al.
Accounts of chemical research, 43(3), 440-454 (2009-12-17)
Meldrum's acid (2,2-dimethyl-1,3-dioxane-4,6-dione) is a molecule with a unique history, owing to its originally misassigned structure, as well as a unique place among acylating agents, owing to its high acidity and remarkable electrophilicity. In this Account, we outline the work
Songlei Zhu et al.
Molecules (Basel, Switzerland), 17(12), 13856-13863 (2012-11-24)
A series of 4-aryl-6-methyl-3,4-dihydro-2H-pyrano[3,2-c]quinolin-2,5(6H)-diones were synthesized via the three-component reactions of aromatic aldehydes, 4-hydroxy-1-methylquinolin-2(1H)-one, and Meldrum's acid catalyzed by L-proline. The structures of the products were identified by spectroscopic analysis. A mechanism for this three-component reaction catalyzed by L-proline was
Davood Nematollahi et al.
Chemical & pharmaceutical bulletin, 58(1), 23-26 (2010-01-05)
Electrochemical oxidation of catechols in the presence of phenyl-Meldrum's acid as a nucleophile in aqueous solution has been studied in detail by means of cyclic voltammetry and controlled potential coulometry. The results indicate that the o-benzoquinone derived from catechols participates
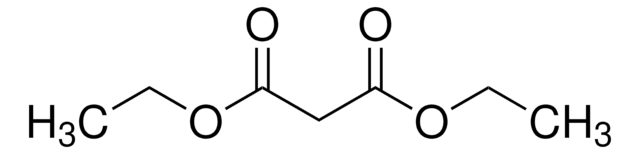
The synthesis of β-keto lactones via cyclization of β-keto ester dianions or the cyclization of Meldrum's acid derivatives.
Lermer L, et al.
Canadian Journal of Chemistry, 70(5), 1427-1445 (1992)
New multicomponent domino reactions (MDRs) in water: highly chemo-, regio-and stereoselective synthesis of spiro {[1, 3] dioxanopyridine}-4, 6-diones and pyrazolo [3, 4-b] pyridines.
Ma N, et al.
Green Chemistry, 12?(8), 1357-1361 (2010)
문서
Knoevenagel Condensation is an organic reaction named after Emil Knoevenagel. It is a classic C-C bond formation reaction and a modification of the Aldol Condensation.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.