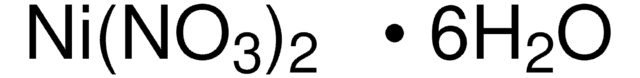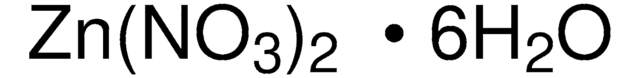203874
Nickel(II) nitrate hexahydrate
99.999% trace metals basis
동의어(들):
Nickel dinitrate hexahydrate, Nickel nitrate hexahydrate, Nickel(2+) dinitrate hexahydrate, Nickelous nitrate hexahydrate
About This Item
추천 제품
Quality Level
분석
99.999% trace metals basis
양식
solid
불순물
≤15.0 ppm Trace Metal Analysis
mp
56 °C (lit.)
density
2.05 g/mL at 25 °C (lit.)
응용 분야
battery manufacturing
SMILES string
O.O.O.O.O.O.[Ni++].[O-][N+]([O-])=O.[O-][N+]([O-])=O
InChI
1S/2NO3.Ni.6H2O/c2*2-1(3)4;;;;;;;/h;;;6*1H2/q2*-1;+2;;;;;;
InChI key
AOPCKOPZYFFEDA-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
애플리케이션
In addition, it can be used:
- As an additive in the fabrication of cellulose acetate polymers(CA) to control the porosity of the polymer. The formation of Ni(NO3)2.6H2O aggregates in the polymer matrix during solidification and the strong interaction between Ni, nitrate, and water molecules results in the formation of well-defined pores on the surface of the CA matrix.
- As a starting material to prepare nickel(ii) Schiff base, which is used as a precursor to synthesize NiO nanoparticles by solid-state thermal decomposition method.
- As a dopant to prepare Ni-Ceria catalyst for selective hydrogenation of acetylene.
- As a catalyst to prepare 3,4-dihydropyrimidinone derivatives via Biginelli cyclocondensation.
특징 및 장점
- Exceptional Purity: The 99.999% purity of Nickel(II) nitrate hexahydrate minimizes contamination from trace metals, ensuring suitability for applications sensitive to even minute impurities.
- Consistent Performance: Ultra-high purity guarantees consistent performance across various applications, reducing variability and enhancing reliability.
- High Purity Standard: Ideal as a standard or reagent for trace metal analysis and high-precision analytical techniques, ensuring accurate and reliable results.
신호어
Danger
Hazard Classifications
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Carc. 1A - Eye Dam. 1 - Muta. 2 - Ox. Sol. 2 - Repr. 1B - Resp. Sens. 1 - Skin Irrit. 2 - Skin Sens. 1 - STOT RE 1 Inhalation
Storage Class Code
5.1B - Oxidizing hazardous materials
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
이미 열람한 고객
문서
Lithium-Ion Battery Performance: Dependence on Material Synthesis and Post‑Treatment Methods
The prevailing strategies for heat and electric-power production that rely on fossil and fission fuels are having a negative impact on the environment and on our living conditions.
Plasmonic nanoparticles have unique optical properties that can be tailored to suit a variety of applications in the biotechnology1–8 and electronics9–16 industries.
Plasmonic nanoparticles have unique optical properties that can be tailored to suit a variety of applications in the biotechnology1–8 and electronics9–16 industries.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.