196436
(1R)-(−)-Fenchone
≥98%
동의어(들):
(−)-1,3,3-Trimethyl-2-norbornanone, (−)-Fenchone, (1R)-1,3,3-Trimethylbicyclo[2.2.1]heptan-2-one
로그인조직 및 계약 가격 보기
모든 사진(2)
About This Item
실험식(Hill 표기법):
C10H16O
CAS Number:
Molecular Weight:
152.23
Beilstein:
2042710
EC Number:
MDL number:
UNSPSC 코드:
12352200
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
≥98%
양식
liquid
광학 활성
[α]24/D −50.5°, neat
refractive index
n20/D 1.461 (lit.)
bp
192-194 °C (lit.)
mp
5-6 °C (lit.)
density
0.948 g/mL at 25 °C (lit.)
작용기
ketone
SMILES string
CC1(C)C2CCC(C)(C2)C1=O
InChI
1S/C10H16O/c1-9(2)7-4-5-10(3,6-7)8(9)11/h7H,4-6H2,1-3H3/t7-,10+/m0/s1
InChI key
LHXDLQBQYFFVNW-OIBJUYFYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
(1R)-(-)-Fenchone is a bridged bicyclic ketone found in fennel oil and thuja oil.
애플리케이션
(1R)-(-)-Fenchone undergoes condensation with pyridinylalkylamines to form chiral iminopyridine ligands, which find applications in enantioselective copper-catalyzed Henry (nitro aldol) reaction. It may also be used in the preparation of enantiopure C(7)-anti-substituted fenchones as new chiral sources.
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Aquatic Chronic 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point (°F)
151.7 °F - closed cup
Flash Point (°C)
66.5 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
이미 열람한 고객
(1R)-(-)-Fenchone.
Bond AD and Davies JE.
Acta Crystallographica Section E, Structure Reports Online, 57(11), o1034-o1035 (2001)
First access to enantiopure C (7)-substituted fenchones: new norbornane-based chiral materials from the chiral pool.
Marti'nez AG, et al.
Tetrahedron Asymmetry, 14(12), 1607-1609 (2003)
Regio-and stereochemical course of the ring expansion of bridged bicyclic ketones to spirocyclic a-keto tetrahydrofurans.
Paquette LA, et al.
The Journal of Organic Chemistry, 57(14), 3956-3965 (1992)
Modular iminopyridine ligands. Application to the enantioselective copper (II)-catalyzed Henry reaction.
Blay G, et al.
Tetrahedron Asymmetry, 17(14), 2046-2049 (2006)
Olga Tzakou et al.
Natural product communications, 4(8), 1103-1106 (2009-09-23)
The essential oils from leaves and inflorescences of L. cariensis Boiss. and L. stoechas L. subsp. stoechas collected in Greece were analyzed by GC and GC/MS. In the inflorescences and leaves essential oils of L. cariensis the most abundant metabolite
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.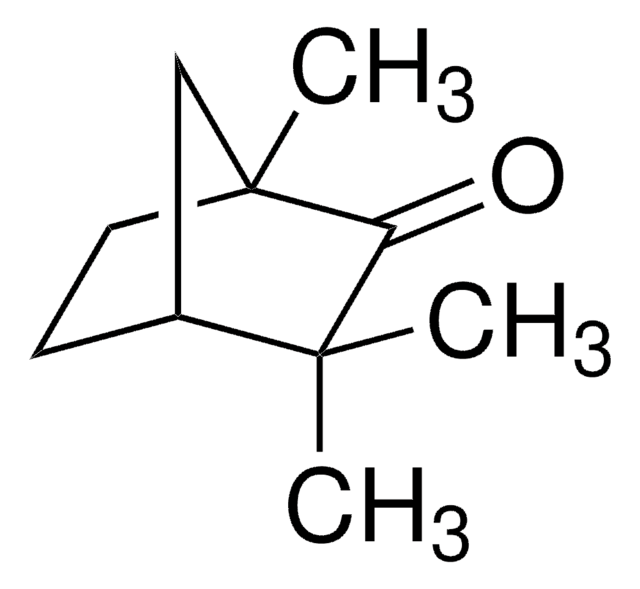
![Poly[(2-ethyldimethylammonioethyl methacrylate ethyl sulfate)-co-(1-vinylpyrrolidone)] average Mw <1,000,000 by GPC, 20 wt. % in H2O](/deepweb/assets/sigmaaldrich/product/structures/334/533/03fcaede-76a6-4b5a-a992-9565ea1ace4a/640/03fcaede-76a6-4b5a-a992-9565ea1ace4a.png)