모든 사진(3)
About This Item
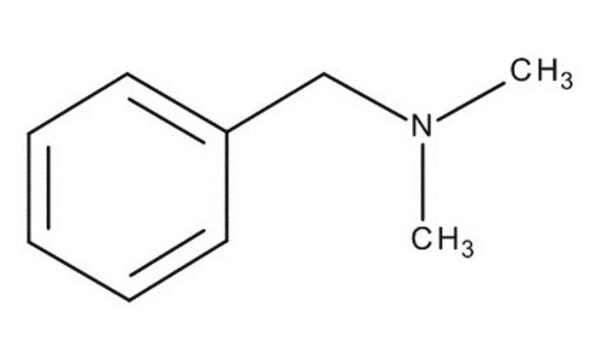
Linear Formula:
C6H5CH2N(CH3)2
CAS Number:
Molecular Weight:
135.21
Beilstein:
1099620
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
≥99%
양식
liquid
refractive index
n20/D 1.501 (lit.)
bp
183-184 °C/765 mmHg (lit.)
mp
−75 °C (lit.)
solubility
water: soluble
density
0.9 g/mL at 25 °C (lit.)
작용기
amine
phenyl
SMILES string
CN(C)Cc1ccccc1
InChI
1S/C9H13N/c1-10(2)8-9-6-4-3-5-7-9/h3-7H,8H2,1-2H3
InChI key
XXBDWLFCJWSEKW-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
관련 카테고리
일반 설명
N,N-Dimethylbenzylamine is commonly used as a reagent in organic synthesis and also serves as a catalyst in the synthesis of polyurethane foams and epoxy resins. It reacts with Os3(CO)12 to form triosmium clusters. Anodic oxidation of N,N-dimethylbenzylamine has been studied in methanol-tetra-n-butylammonium fluoroborate and in methanol-potassium hydroxide.
애플리케이션
N,N-Dimethylbenzylamine was used in the synthesis of bis[(N,N-dimethylamino)benzyl] selenide. It has been used as catalyst during curing reaction of formulations of diglycidyl ether of bisphenol A and tetrahydrophthalic anhydride.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Acute Tox. 4 Dermal - Aquatic Chronic 2 - Eye Dam. 1 - Flam. Liq. 3 - Skin Corr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point (°F)
134.6 °F - closed cup
Flash Point (°C)
57 °C - closed cup
개인 보호 장비
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Products and mechanisms in the anodic oxidation of N, N-dimethylbenzylamine in methanol.
Barry JE, et al.
The Journal of Organic Chemistry, 39(18), 2695-2699 (1974)
Original method for synthesis of chitosan-based antimicrobial agent by quaternary ammonium grafting.
Ernesto Oyervides-Muñoz et al.
Carbohydrate polymers, 157, 1922-1932 (2016-12-19)
Functionalized high molar mass chitosan derivatives with increased antibacterial properties were prepared by the reaction of chitosan with different quaternary ammonium salts. Benzalkonium bromide, pyridinium bromide and triethyl ammonium bromide were synthesized by a quaternization reaction between 1,4-dibromobutane and the
Synthesis, structure, and reactivity of organochalcogen (Se, Te) compounds derived from 1-(N, N-dimethylamino) naphthalene and N, N-dimethylbenzylamine.
Panda A, et al.
Organometallics, 18(10), 1986-1993 (1999)
Wilson Beita-Sandí et al.
Water research, 170, 115323-115323 (2019-12-04)
In this work, we investigated the effect of bromide ion (Br-) on NDMA formation using model precursor compounds, wastewater effluents and surface waters. Previous studies showed that Br- reacts with chloramines and forms bromochloramine, a reactive compound responsible for NDMA
Combined use of sepiolite and a hyperbranched polyester in the modification of epoxy/anhydride coatings: A study of the curing process and the final properties.
Foix D, et al.
Progress in Organic Coatings, 75, 364-372 (2012)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.