추천 제품
양식
liquid
Quality Level
반응 적합성
reagent type: reductant
농도
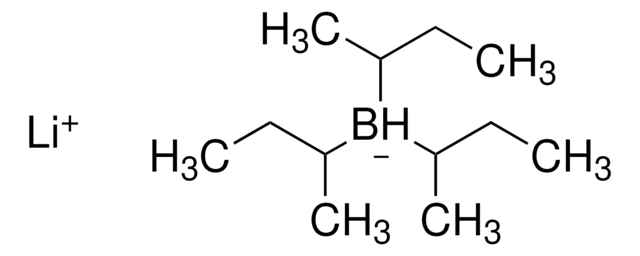
1.0 M lithium triethylborohydride in THF
density
0.892 g/mL at 25 °C
SMILES string
[Li+].[H][B-](CC)(CC)CC
InChI
1S/C6H16B.Li/c1-4-7(5-2)6-3;/h7H,4-6H2,1-3H3;/q-1;+1
InChI key
WCJAYABJWDIZAJ-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Super-Hydride® solution (Lithium triethylborohydride or LiTEBH) is widely used as a powerful and selective reducing agent that shows super hydride activity in organic synthesis.
애플리케이션
LiTEBH can be used as a reagent:
- To reduce alkyl halides to alkanes via dehydrogenation reactions.
- For the selective reduction of epoxides to Markovnikov alcohols.
- To reduce tosylates or mesylates primary alcohols to hydrocarbons.
- For reductive cyclization reactions for the preparation of useful intermediates.
- In the synthesis of hepta(manno-3-deoxy-6-O-t-butyldimethylsilyl)-β-cyclodextrin by reduction of hepta(manno-2,3-anhydro-6-O-t-butyldimethylsilyl)-β-cyclodextrin.
- For hydrodefluorination of C-F bonds using Ni catalyst.
- To prepare alkynyl alcohols from cleavage of cyclic keto-vinyl triflates.
- In the stereoselective reduction of bicyclic imides, isoquinolines, and pyridines.
- To prepare tungsten and molybdenum hydride complexes.
포장
법적 정보
Super-Hydride is a registered trademark of Merck KGaA, Darmstadt, Germany
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Carc. 2 - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B - STOT SE 3 - Water-react 1
표적 기관
Respiratory system
보충제 위험성
Storage Class Code
4.3 - Hazardous materials which set free flammable gases upon contact with water
WGK
WGK 1
Flash Point (°F)
1.4 °F - closed cup
Flash Point (°C)
-17 °C - closed cup
개인 보호 장비
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
이미 열람한 고객
Shin Kamijo et al.
Journal of the American Chemical Society, 128(19), 6499-6507 (2006-05-11)
A thorough analysis of the chemistry of vinylogous acyl triflates provides insight into important chemical processes and opens new directions in synthetic technology. Tandem nucleophilic addition/C-C bond cleaving fragmentation reactions of cyclic vinylogous acyl triflates 1 yield a variety of
Seven-coordinate hydride complexes of molybdenum and tungsten. Crystal and molecular structures of WH(Cl)(CO)2(PMe3)3
Contreras L, et al.
Organometallics, 12(10), 4228-4228 (1993)
Lithium Triethylborohydride
Zaidlewicz M, et al.
Encyclopedia of Reagents for Organic Synthesis, Second Edition, 1-10 (2001)
Tetrahedron Letters, 34, 7239-7239 (1993)
Selective reductions. 32. Structural effects on the reduction of epoxides by lithium triethylborohydride. A kinetic study
Brown HC, et al.
The Journal of Organic Chemistry, 48(18), 3091-3096 (1983)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.