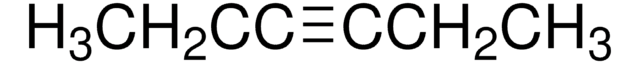161241
1-Phenyl-1-propyne
99%
동의어(들):
(2-Methylethynyl)benzene, 1-Methyl-2-phenylacetylene, 1-Propyn-1-ylbenzene, 1-Propynylbenzene, 3-Phenyl-2-propyne, Methylphenylacetylene, Methylphenylethyne, Phenylmethylacetylene
로그인조직 및 계약 가격 보기
모든 사진(2)
About This Item
Linear Formula:
C6H5C≡CCH3
CAS Number:
Molecular Weight:
116.16
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
99%
양식
liquid
refractive index
n20/D 1.564 (lit.)
bp
185 °C (lit.)
density
0.928 g/mL at 25 °C (lit.)
작용기
phenyl
저장 온도
2-8°C
SMILES string
CC#Cc1ccccc1
InChI
1S/C9H8/c1-2-6-9-7-4-3-5-8-9/h3-5,7-8H,1H3
InChI key
GHUURDQYRGVEHX-UHFFFAOYSA-N
일반 설명
Co-catalyzed reaction between cyclopentene and 1-phenyl-1-propyne has been reported. Bonding properties of 1-phenyl-1-propyne on Cu(111) at 100K have been studied using temperature-programmed desorption and X-ray, ultraviolet and two-photon photoemission spectroscopies. 1-Phenyl-1-propyne is an inhibitor of dopamine beta-hydroxylase. Polymerization of 1-phenyl-1-propyne by TaCl5 and NbCl5 has been reported.
1-Phenyl-1-propyne serves as a starting material for various chemical reactions, such as addition reactions and polymerization.
1-Phenyl-1-propyne serves as a starting material for various chemical reactions, such as addition reactions and polymerization.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point (°F)
143.6 °F - closed cup
Flash Point (°C)
62 °C - closed cup
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
Youngku Sohn et al.
Langmuir : the ACS journal of surfaces and colloids, 23(24), 12185-12191 (2007-10-31)
The bonding properties of 1-phenyl-1-propyne (PP, C6H5CCCH3) on Cu(111) at 100 K have been studied using temperature-programmed desorption (TPD), and X-ray, ultraviolet, and two-photon photoemission spectroscopies (XPS, UPS, and 2PPE). In TPD, there is no evidence for dissociation. Multilayer desorption
Effect of organometallic cocatalysts on the polymerization of 1-phenyl-1-propyne by tantalum pentachloride (TaCl5) and niobium pentachloride (NbCl5).
Masuda T, et al.
Macromolecules, 18(11), 2109-2113 (1985)
Shuhuai Xiang et al.
Journal of the American Chemical Society, 136(16), 5832-5835 (2014-04-12)
Here we report a general method for the measurement of (13)C kinetic isotope effects at natural abundance for reactions that yield two or more products concurrently. We use, as an example, a recently reported Co-catalyzed reaction between cyclopentene and 1-phenyl-1-propyne.
Ekaterina V Pokochueva et al.
Physical chemistry chemical physics : PCCP, 21(48), 26477-26482 (2019-11-30)
Parahydrogen-induced polarization (PHIP) is a powerful technique for studying hydrogenation reactions in gas and liquid phases. Pairwise addition of parahydrogen to the hydrogenation substrate imparts nuclear spin order to reaction products, manifested as enhanced 1H NMR signals from the nascent
G Colombo et al.
The Journal of biological chemistry, 259(24), 15017-15020 (1984-12-25)
The catalytic action of dopamine beta-hydroxylase on 1-phenyl-1-propyne results in concomitant loss of enzyme activity. At pH 5.5 and 25 degrees C, 1-phenyl-1-propyne inactivates dopamine beta-hydroxylase in a mechanism-based fashion. The inactivation rate is first-order, follows saturation kinetics, and is
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.