157910
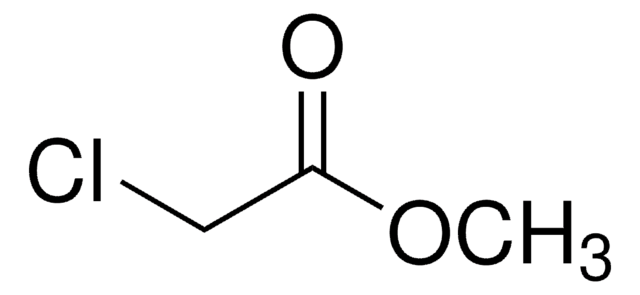
Methyl bromoacetate
97%
동의어(들):
2-Bromoacetic acid methyl ester, Bromoacetic acid methyl ester, Carbomethoxymethyl bromide, Methyl α-bromoacetate, Methyl 2-bromoacetate, Methyl 2-bromoethanoate
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
Linear Formula:
BrCH2COOCH3
CAS Number:
Molecular Weight:
152.97
Beilstein:
506256
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
97%
양식
liquid
refractive index
n20/D 1.458 (lit.)
bp
51-52 °C/15 mmHg (lit.)
density
1.616 g/mL at 25 °C (lit.)
작용기
bromo
ester
SMILES string
COC(=O)CBr
InChI
1S/C3H5BrO2/c1-6-3(5)2-4/h2H2,1H3
InChI key
YDCHPLOFQATIDS-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
관련 카테고리
일반 설명
Methyl bromoacetate is an α-bromo ester. Reactions of the methyl bromoacetate with conjugate base of (methylmethoxycarbene)pentacarbonylchromium(0) yields alkylated carbene complexes.
애플리케이션
Methyl bromoacetate was used in the synthesis of novel coumarins. It was also employed in the synthesis of cis-cyclopropanes.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Oral - Eye Dam. 1 - Skin Corr. 1C - Skin Sens. 1
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point (°F)
147.2 °F - closed cup
Flash Point (°C)
64 °C - closed cup
개인 보호 장비
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
가장 최신 버전 중 하나를 선택하세요:
이미 열람한 고객
Reactions of conjugate bases of metal carbene complexes with expoxides and with a-bromo esters.
Casey CP and Anderson RL.
Journal of Organometallic Chemistry, 73(2), C28-C30 (1974)
Yasameen K Al-Majedy et al.
Molecules (Basel, Switzerland), 19(8), 11791-11799 (2014-08-12)
Some novel coumarins were synthesized starting from 4-hydroxycoumarin and methyl bromoacetate. The structures of the newly obtained compounds were confirmed by elemental analysis, mass, IR and NMR spectra.
One-pot method for stereoselective cyclopropanation of electron-deficient olefins with methyl bromoacetate and phenacyl bromide in the presence of triphenylarsine.
Ren Z, et al.
Synthesis, 2005(16), 2718-2722 (2005)
Hailemichael Ayalew et al.
Polymers, 11(4) (2019-04-13)
Deprotonation-induced conductivity shift of poly(3,4-ethylenedixoythiophene)s (PEDOTs) in aqueous solutions is a promising platform for chemical or biological sensor due to its large signal output and minimum effect from material morphology. Carboxylic acid group functionalized poly(Cn-EDOT-COOH)s are synthesized and electrodeposited on
Josef Dib et al.
Journal of mass spectrometry : JMS, 50(2), 407-417 (2015-03-25)
AdipoR agonists are small, orally active molecules capable of mimicking the protein adiponectin, which represents an adipokine with antidiabetic and antiatherogenic effects. Two adiponectin receptors were reported in the literature referred to as adipoR1 and adipoR2. Activation of these receptors
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.