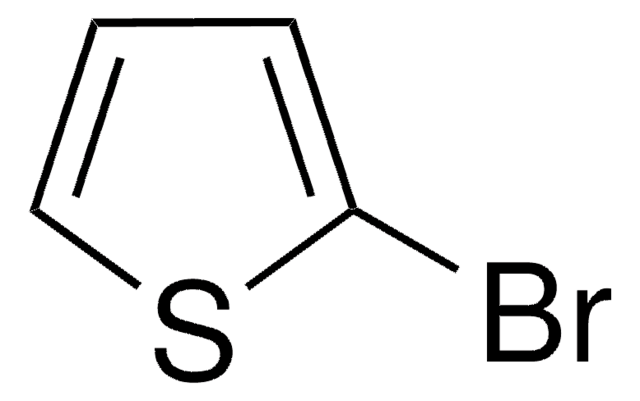
추천 제품
분석
99%
양식
liquid
refractive index
n20/D 1.563 (lit.)
bp
192 °C (lit.)
density
1.172 g/mL at 25 °C (lit.)
작용기
nitrile
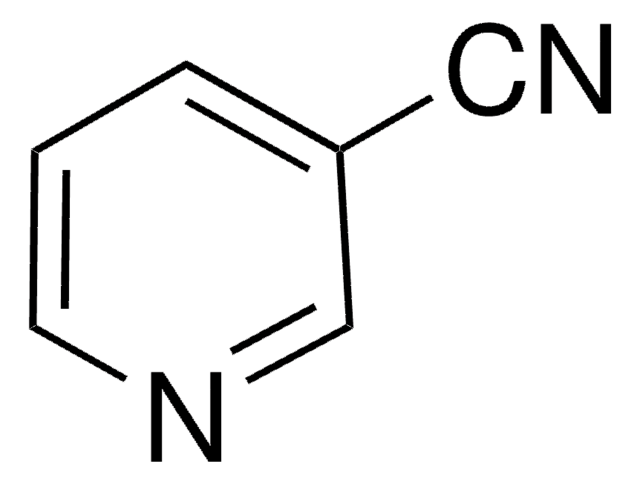
SMILES string
N#Cc1cccs1
InChI
1S/C5H3NS/c6-4-5-2-1-3-7-5/h1-3H
InChI key
CUPOOAWTRIURFT-UHFFFAOYSA-N
관련 카테고리
애플리케이션
2-Thiophenecarbonitrile (2-Cyanothiophene) was used in the preparation of thiaplatinacycles. It was also used in the synthesis of 2,2′-thienylpyrroles.
신호어
Danger
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point (°F)
127.4 °F - closed cup
Flash Point (°C)
53 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
이미 열람한 고객
Ming Yu et al.
Organic letters, 6(6), 1057-1059 (2004-03-12)
[reaction: see text] Two new series of 2,2'-bipyrroles and 2,2'-thienylpyrroles have been prepared by trimethylsilyl trifluoromethanesulfonate (TMSOTf)-mediated reaction of donor-acceptor cyclopropanes with 2-cyanopyrroles and 2-cyanothiophene, respectively. This method opens the door toward a wide variety of unsymmetrical bipyrroles and thienylpyrroles.
Tülay A Ateşin et al.
Inorganic chemistry, 47(11), 4596-4604 (2008-05-02)
The reaction of 2-cyanothiophene with a zerovalent platinum bisalkylphosphine fragment yields two thiaplatinacycles derived from the cleavage of the substituted and unsubstituted C-S bonds. While cleavage away from the cyano group is preferred kinetically, cleavage adjacent to the cyano group
Muhammad Ajmal et al.
Journal of colloid and interface science, 470, 39-46 (2016-03-02)
In this study, the synthesis of micron-sized poly(vinylbenzyl chloride) (p(VBC)) beads and subsequent conversion of the reactive chloromethyl groups to double amidoxime group containing moieties by post modification is reported. The prepared beads were characterized by SEM and FT-IR spectroscopy.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.