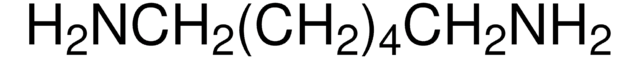15408
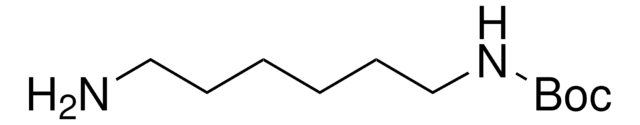
N-Boc-1,3-propanediamine
≥97.0% (GC/NT)
동의어(들):
N-Boc-1,3-diaminopropane, tert-Butyl N-(3-aminopropyl)carbamate
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
Linear Formula:
(CH3)3COCONH(CH2)3NH2
CAS Number:
Molecular Weight:
174.24
Beilstein:
3588328
MDL number:
UNSPSC 코드:
12352116
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
≥97.0% (GC/NT)
반응 적합성
reagent type: cross-linking reagent
refractive index
n20/D 1.454 (lit.)
n20/D 1.459
bp
203 °C (lit.)
mp
22 °C (lit.)
density
0.998 g/mL at 20 °C (lit.)
작용기
Boc
amine
SMILES string
NCCCNC(OC(C)(C)C)=O
InChI
1S/C8H18N2O2/c1-8(2,3)12-7(11)10-6-4-5-9/h4-6,9H2,1-3H3,(H,10,11)
InChI key
POHWAQLZBIMPRN-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
- Tackling vancomycin-resistant bacteria with ′lipophilic–vancomycin–carbohydrate conjugates′: This study discusses the synthesis of derivatives using N-Boc-1,3-propanediamine to develop new antibacterial agents targeting resistant bacterial strains (Yarlagadda et al., 2015).
- Sulfonamides differing in the alkylamino substituent length–Synthesis, electrochemical characteristic, acid-base profile and complexation properties: The study involves N-Boc-1,3-propanediamine in the synthesis of novel sulfonamide derivatives with potential biochemical applications (Ciesielska et al., 2022).
- Direct α-alkylation of primary aliphatic amines enabled by CO2 and electrostatics: Research demonstrating selective α-alkylation of N-Boc-1,3-propanediamine, highlighting a novel method in organic synthesis (Ye et al., 2018).
기타 정보
Synthesis of spermidine analogues; Preparation of pharmacologically active compounds.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point (°F)
228.2 °F - closed cup
Flash Point (°C)
109 °C - closed cup
개인 보호 장비
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
B. Plouvier et al.
Heterocycles, 32, 693-693 (1991)
Inhibition of N8-acetylspermidine deacetylase by active-site-directed metal coordinating inhibitors.
T L Huang et al.
Journal of medicinal chemistry, 35(13), 2414-2418 (1992-06-26)
A number of substrate analogues of N8-acetylspermidine (N8-AcSpd) (16) and chemical modifying agents containing metal coordinating ligands were assayed as inhibitors of the cytoplasmic enzyme N8-AcSpd deacetylase from rat liver. The enzyme is inhibited by metal chelators, several omega-amino-substituted carboxylic
Syota Kawaguchi et al.
The Journal of reproduction and development, 59(3), 219-224 (2013-02-07)
Luteoprotective mechanisms of luteinizing hormone (LH) involved in the maintenance of bovine corpus luteum (CL) function have not been completely clarified. Since antioxidant enzymes are well documented as antiapoptotic factors in the CL of many mammals, we hypothesized that the
Syota Kawaguchi et al.
The Journal of reproduction and development, 59(3), 225-230 (2013-01-30)
Luteinizing hormone (LH) regulates several ovarian functions. However, the luteoprotective mechanisms of LH involved in the maintenance of bovine corpus luteum (CL) function are not well understood. Since prostaglandin F2α (PGF), PGE2 and progesterone (P4) are well documented as antiapoptotic
Yuki Yamamoto et al.
Reproduction, fertility, and development, 28(6), 673-681 (2014-11-06)
Endothelin (EDN) is a possible regulating factor of oviductal motility, which is important for the transport of gametes and embryo. To clarify the factors that control the secretion of EDN in the bovine oviduct, the expression of EDNs, EDN-converting enzymes
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.