추천 제품
Quality Level
제품 라인
ReagentPlus®
분석
99%
양식
solid
mp
256-258 °C (subl.) (lit.)
작용기
ketone
SMILES string
O=C1C2CC3CC(C2)CC1C3
InChI
1S/C10H14O/c11-10-8-2-6-1-7(4-8)5-9(10)3-6/h6-9H,1-5H2/t6-,7+,8-,9+
InChI key
IYKFYARMMIESOX-SPJNRGJMSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
2-Adamantanone on deprotonation in the gas phase affords the corresponding β-enolate anion. It reacts with 1,1-dilithio-1-sila-2,3,4,5-tetraphenylsilole to yield 5-silafulvene.
애플리케이션
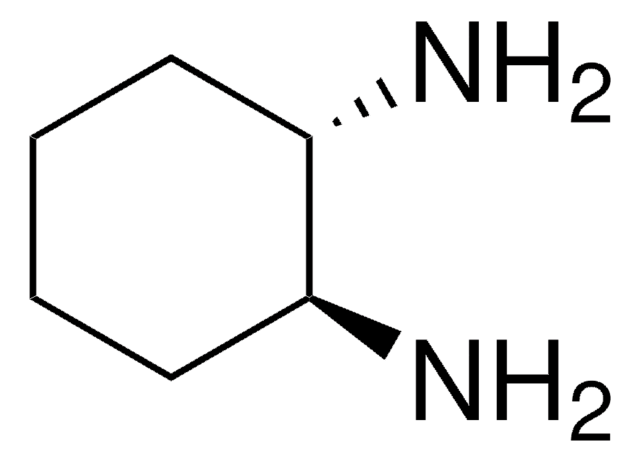
2-Adamantanone was used in the synthesis of dispiro N-Boc-protected 1,2,4-trioxane and (+/-)-1-(adamantan-2-yl)-2-propanamine.
법적 정보
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves
이미 열람한 고객
Sunil Sabbani et al.
Bioorganic & medicinal chemistry letters, 18(21), 5804-5808 (2008-10-11)
Dispiro N-Boc-protected 1,2,4-trioxane 2 was synthesised via Mo(acac)(2) catalysed perhydrolysis of N-Boc spirooxirane followed by condensation of the resulting beta-hydroperoxy alcohol 10 with 2-adamantanone. N-Boc 1,2,4-trioxane 2 was converted to the amine 1,2,4-trioxane hydrochloride salt 3 which was subsequently used
Irina S Toulokhonova et al.
Journal of the American Chemical Society, 126(17), 5336-5337 (2004-04-29)
Reaction of 1,1-dilithio-1-sila-2,3,4,5-tetraphenylsilole with 2-adamantanone produces a 5-silafulvene. This represents a new method for synthesis of silenes, leading to the first example of a silapentafulvene.
Matthew M Meyer et al.
The Journal of organic chemistry, 75(12), 4274-4279 (2010-05-26)
Deprotonation of 2-adamantanone (1) in the gas phase affords the corresponding beta-enolate anion. This ion was independently prepared by the fluoride-induced desilylation of 4-trimethylsilyl-2-adamantanone, and its reactivity and thermodynamic properties were measured (DeltaH degrees(acid) = 394.7 +/- 1.4, EA =
A Corma et al.
Nature, 412(6845), 423-425 (2001-07-27)
The Baeyer-Villiger oxidation, first reported more than 100 years ago, has evolved into a versatile reaction widely used to convert ketones-readily available building blocks in organic chemistry-into more complex and valuable esters and lactones. Catalytic versions of the Baeyer-Villiger oxidation
An adamantanone derivative that is an original modulator of redox processes in the cytochrome P-450 system can serve as an effective remedy against obliterating angiopathy of lower extremities.
I E Kovalev et al.
Doklady. Biochemistry and biophysics, 391, 201-203 (2003-10-09)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.