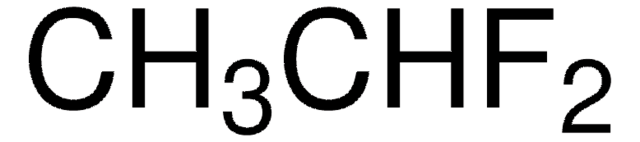All Photos(1)
About This Item
Linear Formula:
CH2F2
CAS Number:
Molecular Weight:
52.02
EC Number:
MDL number:
UNSPSC Code:
12142100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99.7%
bp
−51.6 °C (lit.)
mp
−136 °C (lit.)
density
1.1 g/mL at 25 °C (lit.)
functional group
fluoro
SMILES string
FCF
InChI
1S/CH2F2/c2-1-3/h1H2
InChI key
RWRIWBAIICGTTQ-UHFFFAOYSA-N
Recommended products
Brass hose adapter Z146811 or brass body mini gas regulator Z513539 is recommended.
hose barb
Product No.
Description
Pricing
regulator
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Gas 1 - Press. Gas Compr. Gas
Storage Class Code
2A - Gases
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
M K Ellis et al.
Fundamental and applied toxicology : official journal of the Society of Toxicology, 31(2), 243-251 (1996-06-01)
Difluoromethane (HFC32) is under development as a replacement for chlorofluorocarbons (CFCs) in some refrigeration applications. It has been evaluated by standard studies of toxicity, developmental toxicity, and genotoxicity. In addition, the metabolism and disposition of HFC32 was investigated and a
Heonki Kim et al.
Environmental science & technology, 41(1), 235-241 (2007-02-03)
In this laboratory study, a new experimental method involving the use of a set of four gaseous tracers, was developed for measuring the NAPL saturation directly accessible to the mobile gas as well as the total NAPL saturation in unsaturated
Richard W Denton et al.
Carbohydrate research, 342(12-13), 1624-1635 (2007-07-03)
C-Glycosides in which the pseudoglycosidic substituent is a methylene group have been advertised as hydrolytically stable mimetics of their parent O-glycosides. While this substitution assures greater stability, the lower polarity and increased conformational flexibility in the intersaccharide linker brought about
E T Jensen et al.
The Journal of chemical physics, 129(7), 074703-074703 (2008-12-03)
We have studied the cross section for electron trapping that occurs at the surfaces and interfaces of a variety of thin dielectric films (n-octane, methanol, n-butanol, and difluoromethane) that are grown on Kr buffer films. When such films are bombarded
Nicola Tasinato et al.
The Journal of chemical physics, 136(21), 214302-214302 (2012-06-16)
Difluoromethane (CH(2)F(2), HFC-32) is a molecule used in refrigerant mixtures as a replacement of the more environmentally hazardous, ozone depleting, chlorofluorocarbons. On the other hand, presenting strong vibration-rotation bands in the 9 μm atmospheric window, it is a greenhouse gas
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service