1287722
USP
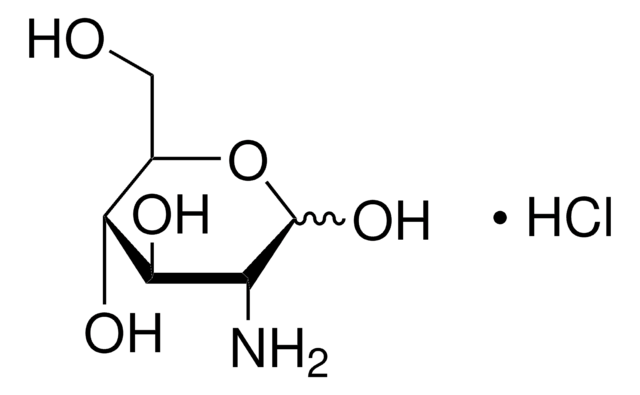
Galactosamine hydrochloride
United States Pharmacopeia (USP) Reference Standard
Synonym(s):
D-(+)-Galactosamine hydrochloride, 2-Amino-2-deoxy-D-galactopyranose hydrochloride, D-Chondrosamine hydrochloride
About This Item
Recommended Products
grade
pharmaceutical primary standard
API family
galactosamine
form
powder
manufacturer/tradename
USP
application(s)
pharmaceutical (small molecule)
format
neat
SMILES string
Cl.N[C@@H](C=O)[C@@H](O)[C@@H](O)[C@H](O)CO
InChI
1S/C6H13NO5.ClH/c7-3(1-8)5(11)6(12)4(10)2-9;/h1,3-6,9-12H,2,7H2;1H/t3-,4+,5+,6-;/m0./s1
InChI key
CBOJBBMQJBVCMW-NQZVPSPJSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Galactosamine hydrochloride is used as a component in carbohydrate analysis related to glycosaminoglycan and heparin quantification. As a USP reference standard, it supports identity and content tests to ensure raw material quality, particularly for complex polysaccharide biologics.
The USP biologics carbohydrates category includes a variety of carbohydrate-based substances that are essential in the development and manufacturing of therapeutic products. These carbohydrates play crucial roles in biological processes and are often utilized as excipients, stabilizers, or active ingredients in pharmaceuticals. The USP provides comprehensive standards, reference materials, and analytical procedures to ensure the identity, quality, purity, and consistency of carbohydrate therapeutics throughout their lifecycle.
The United States Pharmacopeia (USP) provides quality standards for biologics to ensure their safety, efficacy, and quality throughout the manufacturing process. These standards assist manufacturers in adhering to regulatory requirements and help safeguard public health by reducing risks associated with biologics.
For further information and support please go to the website of the issuing Pharmacopoeia.
Application
Other Notes
related product
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documents section.
If you need assistance, please contact Customer Support
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service