1171455
USP
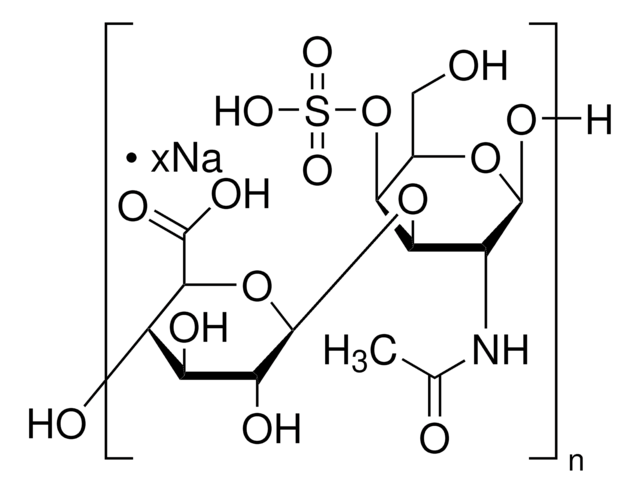
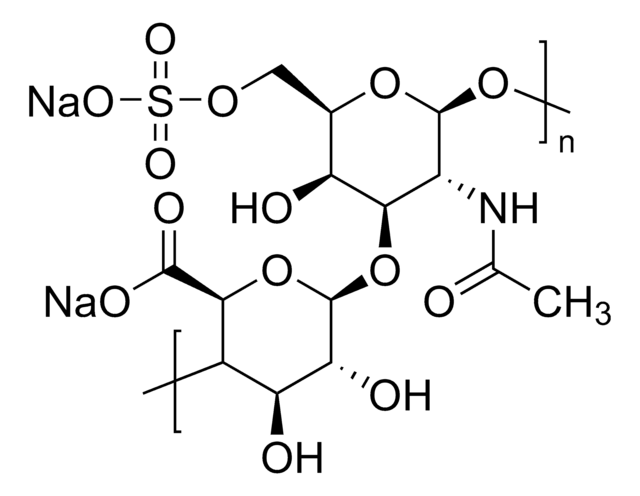
Dermatan sulfate
United States Pharmacopeia (USP) Reference Standard
Synonym(s):
Chondroitin sulfate B sodium salt, β-Heparin, Dermatan sulfate sodium salt
About This Item
Recommended Products
grade
pharmaceutical primary standard
API family
dermatan sulfate
form
solid
manufacturer/tradename
USP
application(s)
pharmaceutical (small molecule)
format
neat
storage temp.
2-8°C
Looking for similar products? Visit Product Comparison Guide
General description
Dermatan sulfate is a sulfated glycosaminoglycan structurally related to heparin, found in animal connective tissues. It plays a role in anticoagulation and cellular signaling. The USP standard is used to characterize dermatan sulfate content and identity in complex glycosaminoglycan mixtures.
The USP biologics carbohydrates category includes a variety of carbohydrate-based substances that are essential in the development and manufacturing of therapeutic products. These carbohydrates play crucial roles in biological processes and are often utilized as excipients, stabilizers, or active ingredients in pharmaceuticals. The USP provides comprehensive standards, reference materials, and analytical procedures to ensure the identity, quality, purity, and consistency of carbohydrate therapeutics throughout their lifecycle.
The United States Pharmacopeia (USP) provides quality standards for biologics to ensure their safety, efficacy, and quality throughout the manufacturing process. These standards assist manufacturers in adhering to regulatory requirements and help safeguard public health by reducing risks associated with biologics.
Dermatan sulfate USP reference standard is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets, has been developed and issued under the authority of the issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia
Application
Further information is available in the monograph, Heparin Sodium, USP43-NF38 - 2195 of the USP (United States Pharmacopeia).
Other Notes
related product
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documents section.
If you need assistance, please contact Customer Support
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service