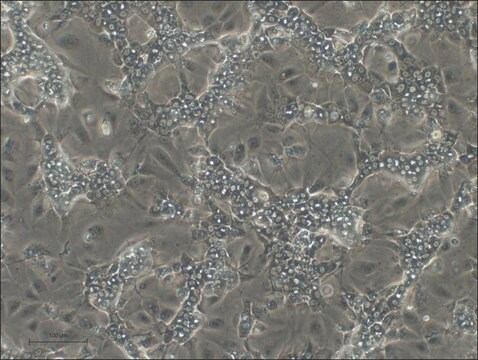
General description
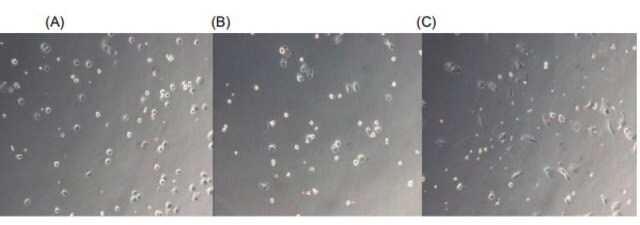
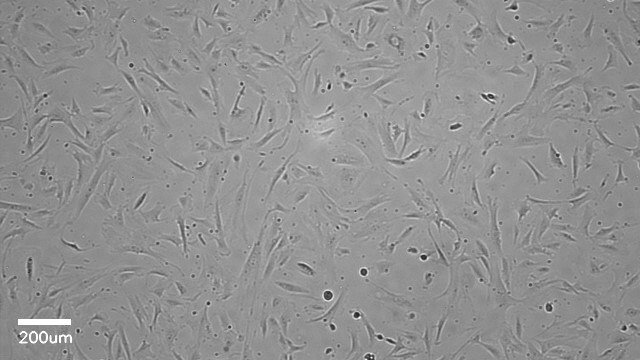
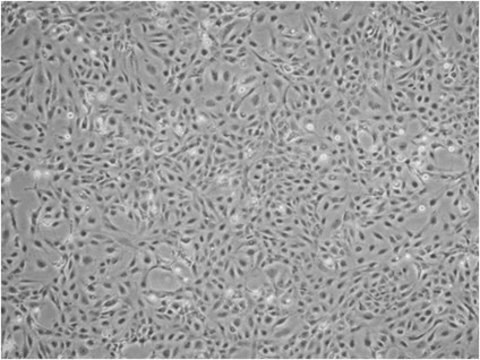
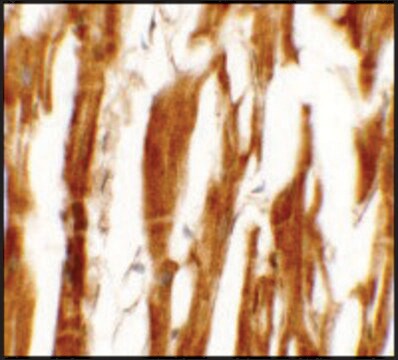
Lot specific orders are not able to be placed through the web. Contact your local sales rep for more details. Hepatic Stellate Cells (HSCs), also called as vitamin A-storing cells, lipocytes, interstitial cells, fat-storing cells, or Ito cells, are liver cells that exist in the space between parenchymal cells and sinusoidal endothelial cells of the hepatic lobule. Stellate cells help store 80% of the vitamin A in the whole body as retinyl palmitate in lipid droplets in the cytoplasm. HSCs are also responsible for vitamin A homeostasis. Primary Hepatic Stellate cells are isolated from whole human livers and are used by researchers to study liver cancer, fibrosis, and liver development and regeneration1. HSCs have a star-like appearance and upon activation can acquire contractile myofibroblast-like morphology, which is an essential step in fibrosis. In fibrosis, activated Stellate cells display a loss of stored vitamin A and increased collagen synthesis, along with other extracellular matrix components.
Application
Stellate cells are ideal in studies of: chronic liver injury, toxicity, activation and differentiation, NAFLD and NASH initiation and progression, fibrosis and more. We offer matched sets of Stellate cells from same donor as Hepatocytes, which can be used for co-culture systems to better simulate the complex microenvironment of the liver and gain insights into various liver-related diseases and conditions.
Features and Benefits
Our cryopreserved hepatic Stellate cells are derived from the human liver, with each donor providing documented consent for research use of non-transplantable organs or tissues. Cells are cryopreserved at the end of the primary culture. Each lot undergoes testing for specific cell markers and comes with a guarantee of ≥70% post-thaw viability. In addition, all cells have been tested for the absence of HIV, HBV, HCV, Syphillis and microbial contaminants (fungi, bacteria, and mycoplasma).
Warning
Each donor is tested and found non-reactive to viral DNA from HIV-1, HIV-2, HTLV I, HTLV II, hepatitis B and hepatitis C. However, no known test can offer complete assurance that the viruses that cause HIV-1, HIV-2, HTLV I, HTLV II, hepatitis B and hepatitis C are not present. Proper precautions and biological containment should be taken when handling cells of human origin, due to their potential biohazardous nature. All human based products should be handled at a BSL-2 (Biosafety Level 2) or higher.
Disclaimer
RESEARCH USE ONLY. This product is regulated in France when intended to be used for scientific purposes, including for import and export activities (Article L 1211-1 paragraph 2 of the Public Health Code). The purchaser (i.e. enduser) is required to obtain an import authorization from the France Ministry of Research referred in the Article L1245-5-1 II. of Public Health Code. By ordering this product, you are confirming that you have obtained the proper import authorization.