General description
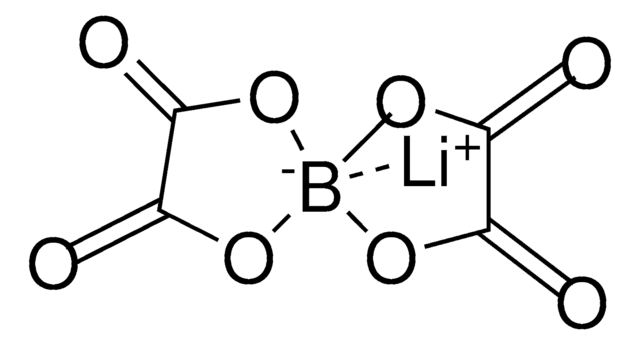
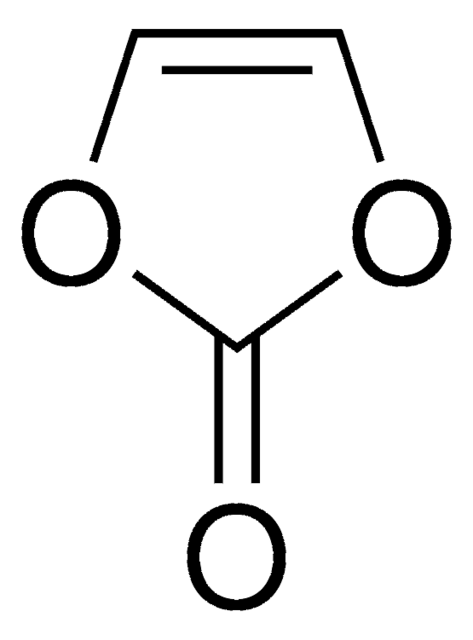
Lithium bis(oxalato)borate (LiBOB) is a lithium salt based on a chelated borate anion. LiBOB is a white powder or crystal that is soluble in many polar organic solvents including THF (500g/L), carbonates, glymes, and lactones(~170g/L). LiBOB is thermally stable to high temperatures (300 °C) before thermally degrading into mild decomposition products (B2O3 and CO2).
Lithium bis(oxalato)borate (LiBOB) is a lithium-boron salt, comes in white powder or crystals and can be used as electrolyte material to enhance the energy efficiency in Lithium ion and lithium polymer batteries. It is a promising salt for Li-ion batteries owing to its unique characteristics such as halide free, non-toxicity and safety. Stability at high temperature and the film-forming characteristics in propylene carbonate (PC)-based electrolyte makes it an excellent additive in electrolyte. We offer high purity LiBOB for the battery research on electrolyte.
Application
LiBOB is commonly used as an electrolytic salt and as an additive in lithium ion and lithium polymer batteries because of its solubility in battery solvents, stability over a wide electrochemical window, and ability to form a stable, conductive solid electrolyte interphase (SEI) layer on many different electrodes. LiBOB has several advantages—such as high thermal stability, halide-free composition, and non-toxic decomposition products—that make LiBOB attractive as a safer alternative compared to traditional fluorinated compounds like LiPF6 and LiBF4, especially in batteries operating in environments with elevated temperatures. Recently, studies have found that LiBOB performs well in lithium metal batteries and as a co-electrolyte with LiTFSI in carbonate or ethereal solvents. The LiBOB-LiTFSI dual-salt electrolyte enhances the cycling stability and capacity retention by forming a robust and conductive SEI on the lithium metal anode. Importantly, LiBOB and LiBOB-LiTFSI dual-salt electrolytes also perform well with lithium nickel manganese cobalt oxide cathode materials, enabling high-voltage (4V) NMC-based Li-metal batteries with improved capacity and cyclability.
LiBOB is a novel boron based Li salt electrolyte material for Li ion batteries. It is environmentally friendly with good film forming property and high thermal stability and is compatible with a variety of anodes and metal oxide cathode.
Lithium bis(oxalate)borate is a conductive salt which can be used in high performance batteries like lithium batteries, lithium-ion batteries and lithium polymer batteries. The halide-free product may be used instead of traditional fluorinated compounds like LiPF6, LiBF4, Li-triflate, methanides, imides etc. In a recent research, Li1.2Mn0.56Ni0.16Co0.08O2 was studied as a cathode material for advanced Li-ion batteries in standard electrolyte solutions with and without LiBOB at 30- and 45-degrees C and it was observed that when these cathodes were tested with LiBOB as an additive in solution, a capacity retention of 98% could be demonstrated during 50 cycles due to a unique stabilizing effect of the additive against 86% in standard electrolyte. Its presence in solutions suppresses the irreversible charge required to activate this cathode material. When cycled at 45 degrees C, the capacity retention of Li1.2Mn0.56Ni0.16Co0.08O2 cathodes in LiBOB containing solutions may reach 97% during 50 cycles as compared to 78% in standard electrolyte solutions. It can also be used as an oxidative additive to prevent the unwanted electrolyte decomposition on the surface of Li1.17Ni0.17Mn0.5Co0.17O2 cathodes. During investigation, it was found that LiBOB additive mitigates severe oxidative decomposition of LiPF6-based electrolytes. Noticeable improvements in the cycling stability and rate capability of Li1.17Ni0.17Mn0.5Co0.17O2 cathodes are achieved in the LiBOB-added electrolyte. After 100 cycles at 60 degrees C, the discharge capacity retention of the Li1.17Ni0.17Mn0.5Co0.17O2 cathode was 28.6% in the reference electrolyte, whereas the LiBOB-containing electrolyte maintained 77.6% of its initial discharge capacity. Moreover, the Li1.17Ni0.17Mn0.5Co0.17O2 cathode with LiBOB additive delivered a superior discharge capacity of 115 mAh g(-1) at a high rate of 2 C compared with the reference electrolyte. The OCV of a full cell charged in the reference electrolyte drastically decreased from 4.22 V to 3.52 V during storage at 60 degrees C, whereas a full cell charged in the LiBOB-added electrolyte exhibited superior retention of the OCV.