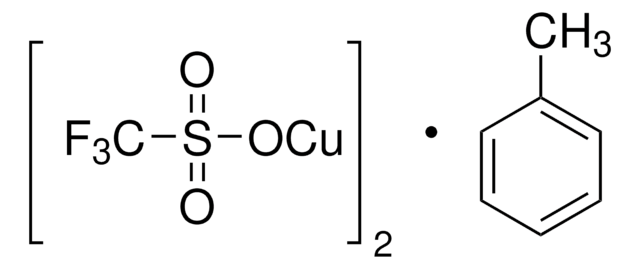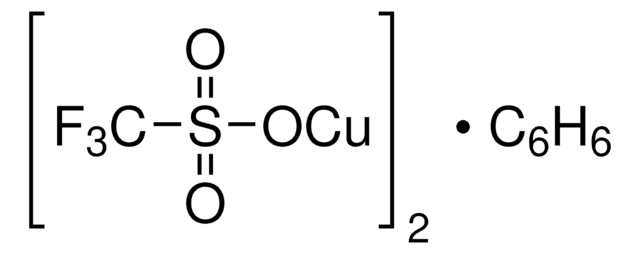682500
Copper(I) thiophene-2-carboxylate
Synonym(s):
(2-Thiophenecarboxylato)copper, 2-Thiophenecarboxylic acid, copper complex, Cu (TC), Cuprous 2-thiophenecarboxylate
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C5H3CuO2S
CAS Number:
Molecular Weight:
190.69
MDL number:
UNSPSC Code:
12161600
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
form
solid
Quality Level
reaction suitability
core: copper
reaction type: C-C Bond Formation
reagent type: catalyst
SMILES string
[Cu]OC(=O)c1cccs1
InChI
1S/C5H4O2S.Cu/c6-5(7)4-2-1-3-8-4;/h1-3H,(H,6,7);/q;+1/p-1
InChI key
SFJMFSWCBVEHBA-UHFFFAOYSA-M
Application
Copper(I) thiophene-2-carboxylate can be used as a reactant or reagent in:
- Studies of xenobiotic response to herbicide safener derivatives.
- Synthesis of functionalized BODIPY dye analogs.
- Orthogonal cross-coupling reactions.
- Preparation of parent borondipyrromethene system.
- Copper mediated cross-coupling.
Reactant or reagent involved in:
- Studies of xenobiotic response to herbicide safener derivatives
- Synthesis of functionalized BODIPY dye analogs
- Orthogonal cross-coupling reactions
- Preparation of parent borondipyrromethene system
- Copper mediated cross-coupling
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Xenobiotic responsiveness of Arabidopsis thaliana to a chemical series derived from a herbicide safener.
Skipsey M, et al.
The Journal of Biological Chemistry, 286(37), 32268-32276 (2011)
The smallest and one of the brightest. Efficient preparation and optical description of the parent borondipyrromethene system.
Arroyo I J, et al.
The Journal of Organic Chemistry, 74(15), 5719-5722 (2009)
Regioselective Syntheses of 2, 3?Substituted Pyridines by Orthogonal Cross?Coupling Strategies.
Koley M, et al.
European Journal of Organic Chemistry, 2011(10), 1972-1979 (2011)
2?and 3?Monohalogenated BODIPY Dyes and Their Functionalized Analogues: Synthesis and Spectroscopy.
Leen V, et al.
European Journal of Organic Chemistry, 2011(23), 4386-4396 (2011)
N-Amidation by copper-mediated cross-coupling of organostannanes or boronic acids with O-acetyl hydroxamic acids.
Zhang Z, et al.
Organic Letters, 10(14), 3005-3008 (2008)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![(R)-1-[(SP)-2-(Diphenylphosphino)ferrocenyl]ethyldicyclohexylphosphine ≥97%](/deepweb/assets/sigmaaldrich/product/structures/245/493/2ae2dd8a-65cc-4aba-9a1f-1292eb1ad8e0/640/2ae2dd8a-65cc-4aba-9a1f-1292eb1ad8e0.png)