Jacobsen Thioureas
Jacobsen’s group has developed a range of chiral thioureas that are versatile and effective organocatalysts. A range of latent nucleophiles can be added to mostly imine-type electrophiles in excellent enantiomeric excesses and, in general, with a broad substrate scope.
The thiourea organocatalyst depicted in Figure 1 was reported by Jacobsen to have a very broad scope in the Strecker reaction (Scheme 1).1 Both aldimines and ketonimines underwent hydrocyanation with very high enantioselectivities in the presence of 1 mol % of the catalyst. Similarly, imine hydrophosphonylation occurred in the presence of 10 mol % of the catalyst (Scheme 2).2 The reaction was particularly effective with electron-withdrawing ester substituents on the phosphite, and was tolerant of a wide variety of aldimines.
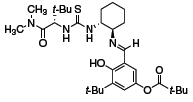
Figure 1 (693421)
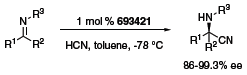
Scheme 1.(693421)
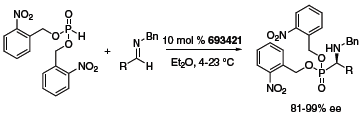
Scheme 2 (693421)
Mannich reactions of Boc-protected imines have also been reported by the Jacobsen group.3 A wide variety of N-Boc arylimines underwent addition with good to excellent yields and enantioselectivities when catalyzed by thiourea organocatalysts (Scheme 3). Again the organocatalytic reaction shows excellent substrate tolerance, particularly for heterocyclic substrates.
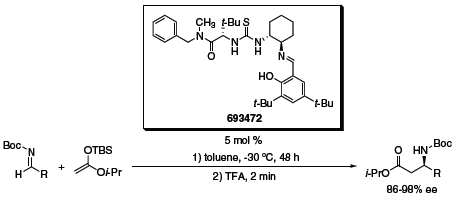
Scheme 3 (693472)
More recently, the cyanosilylation of ketones has also been achieved using a slightly different version of the thiourea organocatalyst (Scheme 4).4 The reaction proceeds for ketones and aldehydes as well in high yields and enantiomeric excesses. In addition, the catalyst can be recovered in near quantitative yield by silica gel chromatography.
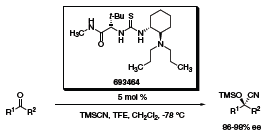
Scheme 4 (693464)
The Jacobsen group has also developed the thiourea organocatalyst depicted in Figure 2 and applied it in the acyl-Pictet–Spengler reaction to form tetrahydro-β-carbolines (Scheme 5).5 This same organocatalyst is also effective in the acyl-Mannich reaction, providing a route to enantioenriched heterocycles from aromatic starting materials and trichloroethyl chloroformate (TrocCl) (Scheme 6).6
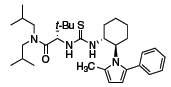
Figure 2 (693340)

Scheme 5 (693340)
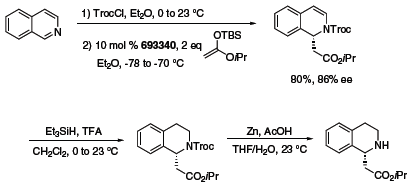
Scheme 6.(693340)
To continue reading please sign in or create an account.
Don't Have An Account?