N8513
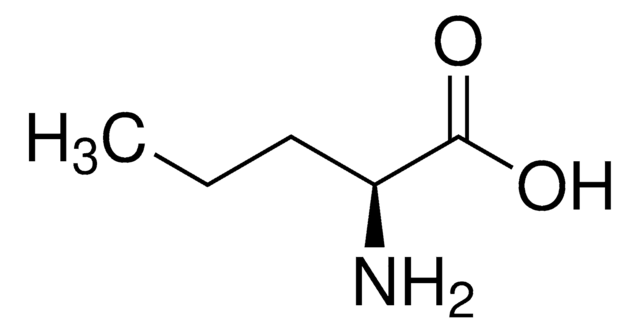
L-Norleucine
suitable for amino acid analysis, BioReagent
Synonym(s):
(S)-(+)-2-Aminohexanoic acid, (S)-2-Aminocaproic acid
Sign Into View Organizational & Contract Pricing
Select a Size
All Photos(1)
Select a Size
Change View
About This Item
Linear Formula:
CH3(CH2)3CH(NH2)CO2H
CAS Number:
Molecular Weight:
131.17
Beilstein:
1721750
EC Number:
MDL number:
UNSPSC Code:
12352209
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
Product Name
L-Norleucine, suitable for amino acid analysis, BioReagent
product line
BioReagent
Quality Level
form
powder or crystals
color
white
mp
>300 °C (lit.)
suitability
suitable for amino acid analysis
application(s)
detection
SMILES string
CCCC[C@H](N)C(O)=O
InChI
1S/C6H13NO2/c1-2-3-4-5(7)6(8)9/h5H,2-4,7H2,1H3,(H,8,9)/t5-/m0/s1
Looking for similar products? Visit Product Comparison Guide
Application
Highly purified internal standard for all amino acid analysis methods.
Other Notes
Non-essential amino acid.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Jan Pícha et al.
Journal of enzyme inhibition and medicinal chemistry, 26(2), 155-161 (2010-06-29)
Ligands containing bulky aliphatic P1 residues exhibit a high affinity towards cytosolic leucine aminopeptidase, a bizinc protease of biomedical significance. According to this specificity, a series of phosphonic and phosphinic compounds have been put forward as novel putative inhibitors of
Haiyan Wei et al.
The journal of physical chemistry. B, 114(36), 11820-11826 (2010-08-24)
Following the studies of urea denaturation of β-hairpins using molecular dynamics, in this paper, molecular dynamics simulations of two peptides, a 35 residue three helix bundle villin headpiece protein HP-35 and its doubly norleucine-substituent mutant (Lys24Nle/Lys29Nle) HP-35 NleNle, were undertaken
L M FitzGerald et al.
Australian veterinary journal, 89(3), 95-100 (2011-02-18)
Four dogs presented with clinical signs of severe hepatic disease after consuming a commercial camel meat diet. Laboratory investigation revealed evidence of severe liver disease, including markedly increased serum alanine aminotransferase (ALT) activity and total bilirubin concentration, and prolonged clotting
Yu-Ying Yang et al.
Chemistry & biology, 17(11), 1212-1222 (2010-11-26)
The advances in bioorthogonal ligation methods have provided new opportunities for proteomic analysis of newly synthesized proteins, posttranslational modifications, and specific enzyme families using azide/alkyne-functionalized chemical reporters and activity-based probes. Efficient enrichment and elution of azide/alkyne-labeled proteins with selectively cleavable
Troy Cellmer et al.
Proceedings of the National Academy of Sciences of the United States of America, 108(15), 6103-6108 (2011-03-29)
Determining the rate of forming the truly folded conformation of ultrafast folding proteins is an important issue for both experiments and simulations. The double-norleucine mutant of the 35-residue villin subdomain is the focus of recent computer simulations with atomistic molecular
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service