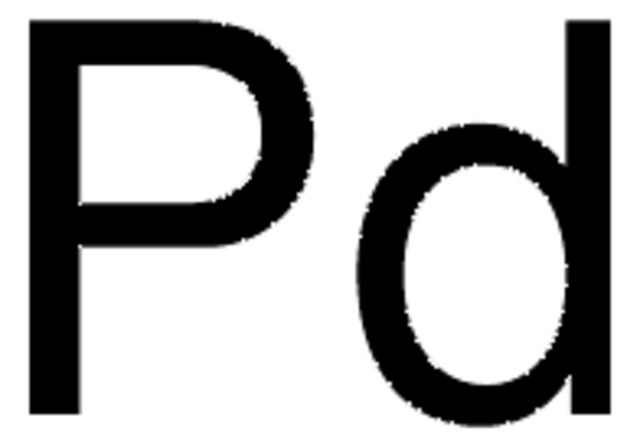691399
Chlorosulfonyl isocyanate
Arxada quality, 99.0-100.3 % (w/w) (T)
Synonym(s):
N-Carbonylsulfamyl chloride, CSI
Sign Into View Organizational & Contract Pricing
Select a Size
All Photos(1)
Select a Size
Change View
About This Item
Linear Formula:
ClSO2NCO
CAS Number:
Molecular Weight:
141.53
Beilstein:
1237247
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor pressure
5.57 psi ( 20 °C)
Quality Level
form
liquid
quality
Arxada quality
manufacturer/tradename
Arxada AG
concentration
99.0-100.3 % (w/w) (T)
impurities
≤1.00% chloropropylsulfonyl isocyanate
refractive index
n20/D 1.447 (lit.)
bp
107 °C (lit.)
mp
−44 °C (lit.)
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- Pharmaceutical intermediates: A study explored the synthesis and carbonic anhydrase inhibitory properties of sulfamides derived from Chlorosulfonyl isocyanate, demonstrating its potential in the development of new therapeutic agents, particularly for treating conditions involving carbonic anhydrase enzymes (Aksu et al., 2013).
- Stereochemical synthesis applications: Chlorosulfonyl isocyanate was utilized for the stereoselective amination of chiral benzylic ethers in the total synthesis of (+)-sertraline, showcasing its critical role in the precise construction of complex pharmaceuticals (Lee et al., 2011).
- One-pot synthetic transformations: The compound′s use in one-pot conversion of trimethylsilyl ethers into urethanes was highlighted, further applied to synthesize novel neuromodulators like carisbamate. This illustrates Chlorosulfonyl isocyanate′s versatility in facilitating efficient and innovative synthetic routes in medicinal chemistry (Dong et al., 2008).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Resp. Sens. 1 - Skin Corr. 1B - Skin Sens. 1
Supplementary Hazards
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
>230.0 °F
Flash Point(C)
> 110 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
C P Sharma et al.
Biomaterials, medical devices, and artificial organs, 12(3-4), 215-233 (1984-01-01)
Natural rubber with C = C bonds had been modified by reaction with chlorosulfonyl isocyanate (CSI) and 70% of the products were obtained, which yielded polyelectrolyte on treatment with NaOH, having sulfamate and carboxylate groups. The polyelectrolyte showed anticoagulant activity.
G Crassous et al.
Biomaterials, 5(3), 153-156 (1984-05-01)
In this paper we report the preparation of a new asymmetric semipermeable styrene-isoprene-styrene block-copolymer membrane. Its modification by addition of gaseous N-chlorosulphonylisocyanate alters neither its permselectivity nor its water permeability rate. This modified membrane possesses an antithrombic activity which depends
J A Picard et al.
Journal of medicinal chemistry, 39(6), 1243-1252 (1996-03-15)
Several series of acyl-CoA:cholesterol O-acyltransferase inhibitors were prepared by the stepwise addition of nitrogen, oxygen, and sulfur nucleophiles to N-chlorosulfonyl isocyanate. The (aminosulfonyl)ureas 3-44 were the most potent inhibitors in vitro, with several compounds having IC50 values < 1 microM.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service